Introduction:
An 86-year-old woman presented to the emergency room at 20:15 after being found down in her home by her son. He noticed she appeared confused with garbled speech, and diminished left arm movement. The patient was last seen normally at 19:00. Her pertinent past medical history included hypertension and chronic obstructive pulmonary disease. On the general exam, except for a heart rate of 110 that was irregular, her vital signs were within normal limits. She was resting comfortably in a stretcher, had no signs of head trauma, had clear breath sounds with no heart murmurs. Her neurological exam revealed that she was alert, and oriented to her name and age but inattentive. She was able to follow simple commands, name objects, and read and repeat short sentences. Her cranial nerve exam was relevant for left homonymous hemianopsia, a forced right gaze, and mild flattening of her left nasolabial fold with preserved strength of the forehead. The rest of her neurological exam was notable for the Medical Research Council (MRC) scale of 4/5 strength, as well as decreased sensation to all modalities in the left arm and leg. No simultagnosia to visual or tactile stimuli was found.
-Where would you localize the lesion?
-What is the differential diagnosis?
Section 1:
Our findings suggest involvement of the saccadic or smooth pursuit pathways (e.g., gaze deviation), visual pathway distal to the optic chiasm (e.g, homonymous hemianopsia), primary motor cortex or both corticospinal and corticobulbar tracts (e.g., left hemiparesis), and both spinothalamic tract and posterior column-medial lemniscal pathway or their fibers from the thalamus to the primary sensory cortex (e.g., left face and arm decreased sensation). A single lesion in the right posterior limb of the internal capsule extending to the thalamus would impair motor and sensory fibers explaining both the left hemiparesis and the left face and arm numbness, but the right gaze deviation or left homonymous hemianopsia would remain unaccounted for. Right gaze deviation could be explained by either hypo- or hyper-function of the right and left cortical eye fields (e.g., frontal cortex and/or posterior parietal cortex), respectively. Left homonymous hemianopsia can arise after lesions in the optic tract, lateral geniculate body, both inferior and superior optic radiations, and primary visual cortex (e.g., occipital cortex). Hence the most likely localization is either a large right hemispheric lesion or multiple smaller right cortical and subcortical lesions.
The differential diagnosis for hyperacute diseases in Neurology mainly consists of vascular, traumatic, epileptic, and some primary headache disorders. There was no history or stigmata of trauma. There was no evidence of any prior history or on-going signs of seizure activity (e.g., tongue biting, incontinence, depressed consciousness level, eyelid flickering). There also was no prior history or evidence of current headache. In the absence of headache or frank obtundation, ruptured aneurysmal subarachnoid hemorrhage is unlikely. The most likely diagnosis is therefore either ischemic or hemorrhagic stroke.
-What are the appropriate immediate next steps?
-What additional workup would you pursue?
Section 2:
Her head computed tomography (CT) did not reveal a hemorrhage or large areas of hypodensity. No early ischemic changes were identified. After ruling out contraindications and conducting the informed consent process, intravenous alteplase 0.9 mg/kg was administered for thrombolysis. Head and neck computed tomography angiography (CTA) revealed a proximal right middle cerebral artery occlusion. She was determined to be a good candidate for mechanical thrombectomy. After successful reperfusion with stent retriever and aspiration, she achieved an eTICI score of 2b67, indicating reperfusion of reperfusion of 67-89% of the territory (Figure 1).
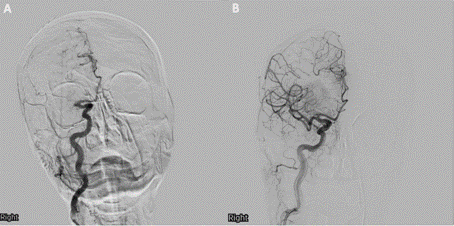
Figure 1 Pre- and post-mechanical thrombectomy cerebral angiogram Coronal view of cerebral angiogram (A) pre- and (B) post-mechanical thrombectomy. (A) reveals right proximal middle cerebral artery (M1) occlusion. (B) shows successful right M1 mechanical thrombectomy with residual parietal occlusion of two distal (M4) branches with complete and fast reconstitution to anterior cerebral artery leptomeningeal collaterals.
Post-stroke workup electrocardiogram revealed new-onset atrial fibrillation. Transthoracic echocardiogram revealed a normal left ventricular ejection fraction with an enlarged left atria, but no atrial septal defect of patent foramen ovale. Laboratory workup revealed a normal Thyroid Stimulating Hormone (TSH), a normal glycated hemoglobin of 5.2%, and a low-density lipoprotein cholesterol of 60.
Post-mechanical thrombectomy, the neurological exam revealed an alert, attentive, and oriented patient with midline gaze, a subtle left inferior homonymous quadrantanopia, full facial strength, MRC scale 4+/5 in the left arm and leg, and normal sensation on the left hemi-body. Forty-eight hours post-mechanical thrombectomy, the patient started complaining of involuntary movements (Video).
-How would you characterize the movements?
-If there is a culprit lesion, where is it?
Section 3:
The phenomenology is defined as involuntary, hyperkinetic, left hemi-body movements. They are rapid, brief, unsustained, and irregular movements that appear to flow from one body part to another restricted to the left hemi-body. The patient is unable to suppress them, but they are painless and unbothersome. On further evaluation, these movements disappear during sleep. This movement disorder is consistent with left hemi-chorea. On later sequences of the video, the left foot appears to hold an ankle inversion posture, which could resemble foot dystonia. Dystonia, however tends to appear with goal-directed movement. In this case, the ankle inversion posture appears to arise from the examiner shifting the patient’s attention to the abnormal movements of the left arm.
Brain magnetic resonance imaging (MRI) obtained 72 hours post-stroke revealed small areas of acute ischemic infarction in the right cerebral hemisphere throughout the vascular territory of the middle cerebral artery. No hemorrhage was found. Relevant areas of infarction include those in the primary motor cortex and their descending fibers, including those functionally connected to the putamen (Figure 2).
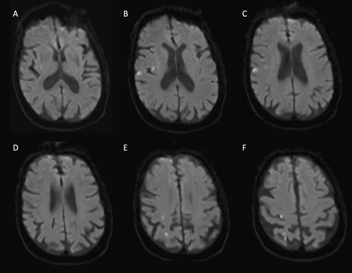
Figure 2 Brain magnetic resonance imaging with punctate acute infarctions. Axial diffusion-weighted imaging with hyperintensities with corresponding apparent diffusion coefficient hypointensities (not shown) in the (A) right posterolateral putamen, (B) posterior insular cortex, (B-F) primary motor cortex, and (E, F) primary somatosensory cortex and somatosensory association cortex.
The patient was started on risperidone 1 mg twice daily with 50% improvement of her symptoms three days after initiating therapy. Therapy was discontinued at a follow-up visit a few weeks later as the chorea had resolved.
Discussion:
Athetosis, chorea, and ballism represent a continuum of involuntary, hyperkinetic movement disorders. While athetosis is a slow form of chorea characterized by writhing movements, ballism is a form of forceful, flinging, high-amplitude, coarse, chorea.(1) The differential diagnosis for chorea is broad, but in acute presentation restricted to a hemi-body distribution, only a few acquired causes need to be considered. In the toxic or metabolic category, diabetic ketoacidosis or non-ketotic hyperosmolar hyperglycemia need to be considered.(2)(3) Our patient, however, had no preceding diabetes mellitus and remained normoglycemic throughout the admission. Chorea as the presenting feature of renal failure or with chronic hepatocerebral degeneration have been described.(4)(5) and these tend to manifest as generalized chorea. Our patient’s renal and hepatic function was normal previously and throughout the hospitalization. Similarly, drug-induced generalized chorea can arise as a tardive syndrome from dopamine depleting therapies or as a manifestation of dopamine repletion therapies (e.g., levodopa); our patient received neither. From vascular causes, hemi-chorea has been described in patients with symptomatic carotid stenosis contralateral to the hemi-choreic side.(6) Our patient had no carotid disease on neck CTA. Post-stroke hemi-chorea is the most likely diagnosis in our patient, which tends to appear as early as within 24 hours.(7) This is earlier in comparison to other post-stroke movement disorders, including dystonia, which tends to appear months after the original insult.(8) This is another clue to distinguishing the nature of the left ankle inversion, as dystonia would not be expected within the acute time frame.
The role of thrombolysis or mechanical thrombectomy in the frequency of post-stroke chorea is unknown. Plegic muscles do not manifest chorea, whereas the degree of weakness in paretic muscles is inversely correlated to chorea severity. Whether successful reperfusion therapy is associated with increased frequency of post-stroke chorea as a byproduct of recovering strength remains to be elucidated.
Multiple areas have been associated with post-stroke chorea at varying frequencies, including the caudate, putamen, globus pallidus, posterolateral and posterior thalamus, temporal lobe, subthalamic nucleus, premotor cortex, and primary and supplementary motor cortex.(7)(9) These heterogeneous localizations, however, localize to a network functionally connected to the posterolateral putamen and appears to be the network that has been affected in our patient.(9)
Treatment of chorea is targeted to identifying and removing the underlying cause, if possible. In post-stroke chorea, treatment should be started if symptomatic and causing the patient significant distress. Medications should be titrated to the lowest efficacious dose, and frequently re-evaluated at interval visits for discontinuation, as post-stroke chorea tends to be self-limiting.(10) Dopamine depleters, reserpine, and antipsychotics have been used. In contrast to classic neuroleptics that block dopamine receptors, drugs that deplete presynaptic dopamine by blocking vesicular monoamine transporter type 2 (VMAT2) seem to be safer and have little or no risk of tardive dyskinesia.(11) VMAT2 inhibitors include (tetrabenazine, deutetrabenazine, and valbenazine).