INTRODUCTION
Earlobe crease (ELC) refers to an acquired linear-shaped fold extending diagonally from the tragus across the earlobe that most often ends in its outer border. From its original description by Sanders T. Frank(1), ELC has been considered a visual skin marker of coronary atherosclerotic disease. While this assumption has been supported by several studies(2)(3)(4)(5)(6), others have found no association between ELC and atherosclerosis involving the coronary or other vascular beds(7)(8)(9). In addition, it has been suggested a link between ELC and cerebrovascular events (either ischemic or hemorrhagic)(10)(11)(12), and a couple of studies proposed a more specific association between ELC and cerebral small vessel disease (cSVD), a common cause of stroke and cognitive decline(13)(14). However, some of these studies included patients recruited from memory clinics or specialized cardiovascular centers, and results may not be extrapolated to the population at large. Should the ELC results be a reliable skin marker of cSVD, it would be of great value for rapid screening of older adults to identify subjects at risk for developing stroke and cognitive decline, particularly in remote settings where the technology needed to diagnose this condition is not readily available(15). Seizing on the prospective Atahualpa Project cohort, we aimed to assess whether ELC is associated with white matter hyperintensities (WMH) severity and progression (used as a biomarker of cSVD) in older adults living in the community.
MATERIALS AND METHODS
Study population and design: The study was carried out in Atahualpa, a rural Ecuadorian village. Characteristics of the study population have previously been detailed(16). Individuals share several characteristics such as race/ethnicity (Amerindian ancestry), levels of education, socioeconomic status, living conditions, and dietary habits.
This study has both a cross-sectional and a longitudinal component. Atahualpa residents aged ≥60 years who had a baseline brain MRI, ELC determinations, and clinical interviews between 2012 and 2019 were eligible for the cross-sectional component of this study, and those who were actively enrolled in the cohort as of May 2021 and had no contraindications for the practice of MRI or were not severely disabled, were invited for the practice of a follow-up MRI (end of study) and were enrolled in the prospective longitudinal component. All individuals signed an informed consent form before the practice of baseline MRI and those who participated in the longitudinal component of the study also signed informed consent before follow-up MRIs. The study was approved by the Ethics Committee of Hospital-Clínica Kennedy, Guayaquil (FWA 00030727).
Earlobe examinations: High-resolution digital photographs of both earlobes were taken with subjects in the sitting position and sent to two independent investigators for ELC identification. Readers were blinded to clinical data and neuroimaging findings. An ELC was considered to be present when the individual has a wrinkle extending from the tragus to the outer border of the earlobe (Figure 1). Individuals with creases related to earrings or distorted earlobe anatomy were not taken into account. Kappa coefficients for the inter-rater agreement were 0.95 for ELC presence; discrepancies were resolved by consensus.
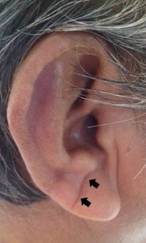
Figure 1 Earlobe crease in a study participant appearing as a wrinkle extending from the tragus to the outer border of the earlobe (arrows).
Neuroimaging studies: MRIs at baseline and follow-up were performed using a Philips Intera 1.5T (Philips Medical Systems, Eindhoven, the Netherlands) following a previously described protocol(16). Interest focused on the severity and progression of WMH, which were defined as lesions appearing hyperintense on T2-weighted images that remained bright on FLAIR (without cavitation) and graded according to the modified Fazekas scale, a widely used visual scale that allows the recognition of none, mild, moderate, and severe WMH(17). MRIs were read by one neuroradiologist and one neurologist blinded to each other’s readings and clinical information. Kappa coefficients for interrater agreement of WMH severity were 0.91 at baseline and 0.93 at follow-up, and discrepancies were resolved by consensus. WMH progression was defined as the increase in at least one grade of the Fazekas scale in the follow-up MRI.
Covariates investigated: Demographics and traditional cardiovascular risk factors were selected as they may have relevance to the association with WMH severity and progression. Risk factors assessment followed the American Heart Association proposed criteria, which stratifies each of the risk factors in the poor range according to well-defined cutoffs: 1) Poor smoking status if the subject is a current smoker or quit <1 year prior; 2) Poor body mass index if ≥30 kg/m2; 3) Poor physical activity if there is no moderate and vigorous activity; 4) Poor diet if there is ≤1 AHA healthy dietary components; 5) Poor blood pressure if ≥140/90 mmHg; 6) Poor fasting glucose if ≥126 mg/dL; and 7) Poor total cholesterol blood levels if ≥240mg/dL(18).
Statistical analysis: In unadjusted univariate analyses, continuous variables were compared by linear models and categorical variables by the chi-square or Fisher exact test, as appropriate. An ordinal logistic regression model was fitted to assess the association between the presence of ELC and WMH severity at baseline, after adjusting for demographics and cardiovascular risk factors. Person time in years was computed utilizing the difference between the baseline and follow-up MRIs. A multivariate Poisson regression model was fitted to estimate the IRR of WMH progression according to the presence of ELC, after taking into account the effect of time as well as the above-mentioned covariates. STATA version 17 (College Station, TX, USA) was used for data analysis.
RESULTS
From 478 community-dwellers aged ≥60 years identified during annual door-to-door surveys carried out from 2012 to 2019, 359 (75%) individuals had a baseline MRI, clinical interviews, and ELC determination, and qualified for the cross-sectional component of the study. Of them, 261 subjects (73%) who also had a follow-up MRI were eligible for the longitudinal component of the study. Figure 2 is a flowchart depicting the enrollment process and the reasons for not including participants at each stage of this study. Of interest, nine individuals who had a follow-up MRI were not included in the longitudinal component of the study because they already had severe WMH at baseline and further WMH progression could not be evaluated. This resulted in 252 individuals in whom the relationship between ELC and WMH progression was assessed. (Figure 2)
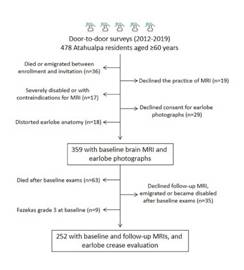
Figure 2 Flow chart depicting enrollment and the number of excluded individuals at each stage of this process.
Cross-sectional component: The mean (±SD) age of 359 study participants at the time of baseline MRI was 67.4±7.6 years (median age: 65.4 years), 205 (57%) were women, 12 (3%) were current smokers, 87 (24%) had a body mass index ≥30 kg/m2, 31 (9%) had poor physical activity, 15 (4%) had an unhealthy diet, 155 (43%) had blood pressure ≥140/90 mmHg, 109 (30%) had fasting glucose ≥126 mg/dL, and 46 (13%) had total cholesterol blood levels ≥240mg/dL. ELC was present in 175 (49%) subjects. On baseline MRI, 107 (30%) participants did not have WMH, 174 (48%) had mild, 56 (16%) had moderate, and 22 (6%) had severe WMH.
Table 1 shows the clinical and neuroimaging characteristics of individuals with and without ELC. As noticed, there were no significant differences across groups. An ordinal logistic regression model that uses ELC presence as the independent variable and grades of WMH severity as the outcome (dependent variable) did not show significant associations between the main variables investigated, after adjusting for clinical covariates (Table 2). In this model, age and poor physical activity remained statistically significant. Because of the important age effect, we fitted an interaction model that failed to show a significant effect modification of age over ELC (OR: 0.99; 95% C.I.: 0.93 - 1.07).
Table 1 Characteristics of Atahualpa residents aged ≥60 years according to the presence or absence of an earlobe crease (unadjusted analysis).
| Earlobe crease absent (n=184) | Earlobe crease present (n=175) | p value | |
|---|---|---|---|
| Age at baseline, years, mean ± SD | 67.1 ± 7.5 | 67.7 ±7.7 | 0.455 |
| Women, n (%) | 110 (59) | 95 (54) | 0.293 |
| Current smoker, n (%) | 6 (3) | 6 (3) | 0.929 |
| Body mass index ≥30 kg/m2, n (%) | 43 (23) | 44 (25) | 0.659 |
| Physical inactivity, n (%) | 18 (10) | 13 (7) | 0.427 |
| Unhealthy diet, n (%) | 8 (4) | 7 (4) | 0.869 |
| Blood pressure ≥140/90 mmHg, n (%) | 77 (42) | 78 (45) | 0.602 |
| Fasting glucose ≥126 mg/dL, n (%) | 57 (31) | 52 (30) | 0.795 |
| Total cholesterol ≥240 mg/dL, n (%) | 27 (15) | 19 (11) | 0.279 |
| White matter hyperintensities | |||
| Fazekas grade 0 (none), n (%) | 49 (27) | 58 (33) | 0.178 |
| Fazekas grade 1 (mild), n (%) | 92 (50) | 82 (47) | 0.551 |
| Fazekas grade 2 (moderate), n (%) | 28 (15) | 28 (16) | 0.838 |
| Fazekas grade 3 (severe), n (%) | 15 (8) | 7 (4) | 0.101 |
Table 2 Ordinal logistic regression model showing a non-significant association between earlobe crease presence and severity of white matter hyperintensities.
| Grades of white matter hyperintensities severity | Odds ratio | 95% confidence interval | p value |
|---|---|---|---|
| Earlobe crease | 0.72 | 0.48 - 1.06 | 0.098 |
| Age | 1.09 | 1.06 - 1.12 | <0.001* |
| Being women | 0.99 | 0.66 - 1.52 | 0.993 |
| Smoking status | 1.21 | 0.39 - 3.74 | 0.745 |
| Body mass index ≥30 kg/m2 | 1.13 | 0.70 - 1.82 | 0.622 |
| Physical inactivity | 2.21 | 1.07 - 4.57 | 0.033* |
| Unhealthy diet | 2.51 | 0.95 - 6.63 | 0.062 |
| Blood pressure ≥140/90 mmHg | 1.23 | 0.81 - 1.87 | 0.323 |
| Fasting glucose ≥126 mg/dL | 1.12 | 0.72 - 1.73 | 0.610 |
| Total cholesterol ≥240 mg/dL | 1.52 | 0.83 - 2.81 | 0.177 |
*Statistically significant result
Longitudinal component: The 252 participants who had both baseline and follow-up MTIs (and ELC assessment) were followed for a mean of 6.5±1.4 years, and the total time of follow-up was 1,640 person-years (95% C.I.: 1,594 - 1,685 years). The mean (±SD) age was 65.4±5.9 years (median age: 63.6 years), 139 (55%) were women, 10 (4%) were current smokers, 60 (24%) had a body mass index ≥30 kg/m2, 11 (4%) had poor physical activity, 11 (4%) had an unhealthy diet, 96 (38%) had blood pressure ≥140/90 mmHg, 71 (28%) had fasting glucose ≥126 mg/dL and 35 (14%) had total cholesterol blood levels ≥240mg/dL. ELC was present in 126 (50%) subjects.
On baseline MRI, 89 participants (35%) did not have WMH, 130 (52%) had mild, and 33 (13%) had moderate WMH. At follow-up, 51 (20%) individuals did not have WMH, 111 (44%) had mild, 67 (27%) had moderate, and 23 (9%) had severe WMH. A total of 103 (41%) individuals had MRI evidence of WMH progression. Progression from none-to-mild WMH was noticed in 33 cases, from none-to-moderate in five, from mild-to-moderate in 42, from mild-to-severe in 10, and from moderate-to-severe in 13.
Of 126 individuals with ELC, 54 had WMH progression as opposed to 49 of the 126 who did not have ELC (43% versus 39%; p=0.522). A Poisson regression model, using ELC as the exposure and WMH progression as the outcome showed no significant association between both variables, after adjusting for demographics, cardiovascular risk factors, and the time between baseline and follow-up MRIs. Only age at baseline MRI reached independent significance in this model (Table 3). Smoking status was not included in the Poisson regression model due to collinearity with other variables.
Table 3 Poisson regression model showing a non-significant association between earlobe crease presence and progression of white matter hyperintensities after a mean of 6.5±1.4 years of follow-up.
| White matter hyperintensities progression | Incidence Rate Ratio | 95% confidence interval | p value |
|---|---|---|---|
| Earlobe crease | 1.02 | 0.69 - 1.51 | 0.923 |
| Age at baseline | 1.04 | 1.01 - 1.07 | 0.007* |
| Being women | 0.94 | 0.62 - 1.41 | 0.763 |
| Body mass index ≥30 kg/m2 | 0.83 | 0.47 - 1.45 | 0.505 |
| Physical inactivity | 0.80 | 0.25 - 2.57 | 0.704 |
| Unhealthy diet | 0.79 | 0.28 - 2.18 | 0.647 |
| Blood pressure ≥140/90 mmHg | 1.05 | 0.70 - 1.59 | 0.799 |
| Fasting glucose ≥126 mg/dL | 0.86 | 0.54 - 1.36 | 0.525 |
| Total cholesterol ≥240 mg/dL | 1.18 | 0.66 - 2.10 | 0.563 |
*Statistically significant result
DISCUSSION
Results of this study suggest that ELC presence is not associated with WMH severity nor related to WMH progression in community-dwelling older adults of Amerindian ancestry. Our findings differ from those reported in cognitively impaired patients from South Korea, in whom ELC was significantly associated with WMH(13). However, the recruited population and the design of the Korean study were vastly different from those of the present study. Likewise, a Turkish study also found an association between ELC and WMH presence (not WMH severity assessed)(14), but analyses were not adjusted for age or any other covariate, and this was an important shortcoming of this study since the frequency of both ELC and WMH increases with age. Another study of patients admitted to a stroke unit showed no differences in the frequency of ELC across the different categories of ischemic stroke subtypes, suggesting that ELC is not specifically linked to cSVD(12).
Besides the above-mentioned studies, there is scant additional information on the association between ELC and WMH, which makes the present study the first to unravel the lack of relationship between both variables and, at the same time, to show the independent significance of increased age on this association in both the ordinal logistic (cross-sectional) and the Poisson regression (longitudinal) models.
Regarding pathogenic mechanisms potentially implicated in the previously proposed link between ELC and cSVD, it has been trying to parallel the suggested mechanisms involved in the association between ELC and atherosclerosis with that of ELC and WMH. ELC has traditionally been proposed as a visual sign of collagen and elastin fibers disintegration of the earlobe and thus, a visible sign of arterial wall disease that could be present in the coronary arteries or other vascular beds. Indeed, an autopsy-based report suggested that pathogenetic mechanisms involved in the evolution of atherosclerosis (related to collagen metabolism) may also occur in the skin(19). In addition, it has been suggested that circulating inflammatory biomarkers (in particular pentraxin-3) and oxidative stress may account for the simultaneous occurrence of ELC and atherosclerosis(20). Other mechanisms implicated in the simultaneous occurrence of ELC and atherosclerosis include endothelial dysfunction(21), shortening of telomere length in peripheral blood cells(22), decreased macrophages activity(23), and low circulating levels of the polypeptide hormones adropin and irisin(24). A novel hypothesis elaborates on the role of the age-suppressing hormone Klotho in the pathogenesis of the association between ELC and atherosclerosis(25). Despite all these assumptions, mechanisms addressing the association between ELC and cardio- or cerebrovascular complications are not fully understood thus far.
It is noteworthy the high frequency of ELC in the study population (49% and 50% for subjects participating in the cross-sectional and longitudinal components of the study, respectively). These percentages are considerably higher than those reported in some but not all studies(13)(14). Differences in frequency can be explained by the ethnic background and the age of the present study population (≥60 years) since it is well-known that ELC frequency increases with age and is more prevalent in certain races/ethnic groups.
Major strengths of our study include the population-based design with an unbiased enrollment of participants as well as the cross-sectional and the longitudinal components, which allowed the evaluation of the association between ELC presence and WMH at baseline as well as the role of ELC on WMH progression after more than six years of follow-up. The fact that Atahualpa residents may not be representative of people living in other settings or belonging to other races/ethnic groups, might be a limitation.