1. Introduction
Body temperature (Tb) is a critical ecophysiological variable affecting the performance of ectotherms because intrinsic aspects of ecology, behavior and physiology are sensitive to Tb (Huey, 1982; Huey & Stevenson, 1979), including reproduction (Adolph & Porter, 1993), foraging (Ayers & Shine, 1997), growth (Kingsolver & Woods, 1997), locomotion (Ojanguren & Brañta, 2000), and courtship (Navas & Bevier, 2001).
Ectotherms can exhibit heliothermy or tigmothermy, obtaining energy by direct exposure to the sun or by direct contact with the substrate, respectively (Garrick, 2008). Tigmothermy has been recognized for species living in tropical rainforests and nocturnal species (Belliure & Carrascal, 2002). For species living in forests, there is a thermal refuge on the effect of the surrounding climate, creating particular microclimate conditions (higher relative humidity and lower temperatures respect to open habitats that allows avoid overheating and dehydration (Gaudio et al., 2017). Thus, some tropical forest ectotherms appear to be relatively passive with respect to environmental temperatures and behave as thermoconformers (Huey & Webster, 1976; Kohlsdorf & Navas, 2006).
The Western Amazon basin (Ecuador, Perú, and Colombia) has the higher diversity of amphibian (Frost, 2023; Vigle, 2008). Near of the Ecuadorian Amazon there are published studies on herpetofauna for several locations. Duellman (1978), showed that the herpetofauna of Santa Cecilia, on the Río Aguarico, Province of Napo, is composed of 173 species; Lescure and Gasc (1986) compared the spatial distribution between assemblages of lizards and anurans along the Río Putumayo and Ampiyacu (Perú), Igaraparana (Colombia), and Santa Cecilia Ecuador); Almendáriz (1987) reported 101 species of amphibians and reptiles of the Province of Pastaza (Ecuador); Duellman and Mendelson (1995) reported 68 amphibian and 46 reptile species north of the Department of Loreto in the Amazonian Perú. Izquierdo et al. (2000) found 34 amphibian and 27 reptile species in the Province of Sucumbios (Ecuador); however, data on the thermal biology of ectotherms in this region are insufficient. We explored the basic thermal biology of three frogs and their ecological implications in northeastern Ecuador. We describe here below the relationships among Tb and microenvironmental temperatures (e.g., Huey & Slatkin, 1976).
2. Materials and Methods
2.1 Study area
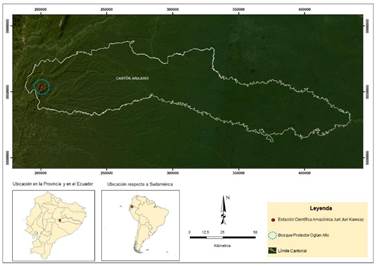
Fieldwork was carried out on July 16th and 17th, 2017, in the surroundings of the Juri-Juri Kawsay Amazon Scientific Station, located in the Protected Forest of Oglán Alto, Arajuno Canton, province of Pastaza, Ecuador (77.688583°N, 01.324152°W, Datum WGS84, elevation 604 m) (Figure 1). The local vegetation is premontane pluvial forest characterized by emergent trees (e.g., Ceiba pentandra, Pachira insignis, Ficus perisiana, and Otoba parviflora, among others); as well as abundant mosses and liverworts in the leaves and branches of the arboreal and shrubby vegetation (Cerón Martínez et al., 2007). Mean annual temperature is 18-24 °C, and annual precipitation ranges between 4,000 and 8,000 mm per year (Cerón Martínez et al., 2007).
2.2 Study species
Dendropsophus bifurcus (Figure 2-A) is distributed from the northwestern part of the Amazon Basin in Colombia, Ecuador and northern Perú (Jungfer et al., 2010). The native range of Rhinella marina (Figure 2-B) extends from the East Andes to Central Amazonia (Acevedo et al., 2016) although they have established populations in Australia, Eastern Asia and several islands of the Caribbean and the Pacific, as the result of translocations by humans (Lever, 2001); Scinax ruber (Figure 2-C) is widely distributed throughout the Guianas and Amazonia (Fouquet et al., 2007).
2.3 Body temperature
We collected 11 D. bifurcus, 17 R. marina, and 37 S. ruber (listening and following the direction of their songs, or through direct search in potential microhabitats: e.g. along water reservoirs, on stems, leaves, leaf litter, and logs, principally), by hand from 1,800 to 2,300 h (all individuals were active at the time of capture). Immediately upon capture, we measured body temperature (Tb), holding tightly on the tarsi to carefully insert a thermocouple into the vent. Air temperature (Ta) was recorded by placing the thermocouple 1 cm above substrate where the individual was first seen, and substrate temperature (Ts) was measured touching the substrate where individual first observed to the nearest 0.1°C with a thermocouple type K connected to quick-reading digital thermometer (Fluke 51-II®). Tb, Ta, and Ts were recorded during the first 5 seconds of the thermometer reading. On both days, the frogs and toads collection preceded intense rains, at temperatures close to 25 °C and relative humidity around 80 %. All organisms that required a capture time > 1 min were excluded from the statistical analyzes.
2.4 Statistical analysis
We used linear multiple regression [MLR] and best subsets regression [BSR] analysis for selecting the variables of MLR by systematically searching through the different combinations of Ta and Ts and selecting the subsets of variables that best contribute to predicting Tb for each species. We used the values of R 2 by BSR as best criterion to establish tigmothermy or heliothermy tendencies: if R 2 was higher between Tb vs Ta it indicates heliothermy, but if R 2 is higher between Tb vs Ts is a tendency to tigmothermy. On the other hand, we use the value of the slopes generates by BSR to establish active thermoregulation, or passive thermoregulation (thermoconformers) tendencies: if Tb vs Ta- Ts is close to zero, the organisms are active thermoregulators. If the value of the slope between Tb vs Ta- Ts is close to one, the organisms are thermoconformers (criterion of Huey & Slatkin, 1976). We used ANOVA and Bonferroni t-test post-hoc to compare Ta and Ts with the three species. To test the difference in Tbs among species, we realized a covariance analysis [ANCOVA], using Ts as covariable.
3. Results
Means Tb, Ta and Ts for each species are detailed in Table 1. The best equations obtained by BMR that explained the thermal relationships were: Tb = 8.39 + 0.74 * Ts (R2 = 0.27, p > 0.05, n = 11); Tb = 4.79 + 0.86 * Ta (R2 = 0.51, p < 0.05, n = 17); and Tb = 2.14 + 0.96 * Ts (R2 = 0.71, p < 0.05, n = 37) for D. bifurcus, R. marina, and S. ruber, respectively.
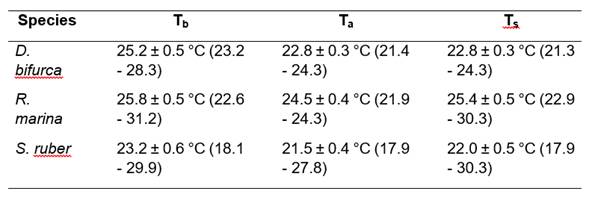
Table 1 Mean Tb, Ta, and Ts for D. bifurcus, R. marina, and S. ruber from Juri-Juri Kawsay Amazon Scientific Station, province of Pastaza, northeastern Ecuador. Means are given ± 1 S.E. In brackets are the minimum and maximum temperatures.
Thermoconformity tendencies are observed in the three species; tigmothermy tendencies are presented by D. bifurcus and S. ruber, and a heliothermy tendency is presented by R. marina. Ts did not present differences between species (ANOVA; F2,65 = 0.84, p > 0.05), while Ta presented significant differences for the three species (ANOVA; F2,65 = 11.39, p < 0.05; Table 2). We observed different Tbs among the species (ANCOVA with Ts as the covariate; F1,44 = 4.16, p < 0.05).
4. Discussion
Dendropsophus bifurcus.-- The mean Tb of D. bifurcus (25.2 ± 0.5 °C; 23.2 - 28.3; n = 11), was within the range of Tbs observed for other Dendropsophus species found at lower elevation, range from 24.8° to 25.8°C (Navas et al., 2013), but higher than those found in the mountain, range from 12.2° to 15.8°C (Navas, 1996). Considering our observations on the Tbs of D. bifurcus, we suggest that it is not different from the other congeners that inhabit tropical sites at low altitudes (≤ 90 m): D. ebraccatus (24.8 °C); D. microcephalus (25.8 °C), and altitude can be a limiting factor to reach Tbs greater than 20 °C: D. labialis (15.8 °C and 12.2 °C at 2,900 meters; 14.7 °C and 10 °C, at 3,500 meters; Navas et al., 2013). Considering the values of BMR, we suggest that D. bifurcus showed tendencies towards thermoconformity and tigmothermy (Huey & Slatkin, 1976).
Rhinella marina. -- The mean Tb for this species (25.8 ± 0.5 °C; 22.6 - 31.2; n = 17), is similar to other populations, range 24.2 - 27 °C, mean 25.2 °C; (Brattstrom, 1963). Under controlled conditions, at a humidity close to 80%, R. marina presented a similar Tb, which could indicate the optimal physiological for this species (Malvin & Wood, 1991). We observed that Tbs were higher than others congeners (R. spinulosa) distributed at different altitudes in the North, Center, and South of Chile: 19.8 °C, near to 2,469 meters; 20.7 °C, at 2,390 meters; and 20.3 °C, at 1,410 meters, respectively (Alveal-Riquelme, 2015); Rhinella arenarum (18.3 °C, around 730 meters) in Argentina (Sanabria et al., 2011); and Rhinella schneideri (20.8 °C, near to 630 meters) in Brazil (Noronha-de-Souza et al., 2015). However, these Tbs fell within the activity ranges, because below 13.7 °C and above 37.4 °C, their locomotion is limited (Kearney et al., 2008). BMR showed trends towards heliothermy and thermoconformity, this trend is similar in invasive populations inhabiting the tropical east coast of Australia (Seebacher & Alford, 2002).
Scinax ruber.-- The mean Tb in this study (23.2 ± 0.6 °C; 18.1 - 29.9; n= 37), was lower that observed for other population: 24.1°C, at 218 meters of elevation (Romero Barreto, 2013), but higher than S. fuscovarius and S. hiemalis distributed at higher altitudes, 22.5 °C, at 1,800 meters, and 12.5 °C, 1,200 meters, respectively (Navas & Araujo, 2000). Our results may suggest that altitude is a determining factor in the Tbs presented by different populations of Sinax frogs, observing a decline in Tbs with elevation at tropical latitudes (Andrews, 1998; Janzen, 1967). BMR showed trends towards tigmothermy and thermoconformity, a tendency similar to S. acuminatus and S. nasicus from Argentina (Novo, 2009).
Species comparison.-- Tbs observed in D. bifurcus, and R. marina were similar, contrary to S. ruber which had a significantly lower Tb. The locomotor performance dependent of Tb can explain these differences, since the best performance has been observed at Tbs close to the one presented by R. marina (Malvin & Wood, 1991), and other Scinax species in similar environments (Navas et al., 2008). In tropical forests, at low elevations, Tbs tend to be stable (Navas et al., 2008), thus the thermoconformity tendency of the three species can obey to variation of few degrees between the coldest and the warmest month in tropical latitudes (Janzen, 1967), shade forest environments (Huey, 1974), and high thermal quality reported for tropical environments (Vickers et al., 2011); while the tigmothermy tendency presented by D. bifurcus and S. ruber is characteristic of shade-dwelling organisms (Ruibal, 1961), and heliothermy presented by R. marina seems a strategy to avoid potential impacts of thermal stressor on physiology, ecology and survival (Narayan & Hero, 2014).
Our results suggest that thermal environment is influencing different thermoregulatory strategies, such as the tigmothermy and heliothermy of frogs and toads distributed in tropical environments at low elevation. Further studies are needed, specifically focused on the effect of both, deforestation at local scale, and climate change at regional scale on these thermoregulatory strategies and performance at different Tbs.
5. Conclusions
The observations on Tbs of D. bifurcus, show that it is not similar to the other congeners that inhabiting tropical sites at low altitudes (≤ 90 m), yet it is higher than the one observed for those from high altitudes (over 2,900 m elevation). Thus, the values of BMR, suggest that D. bifurcus present tendencies towards thermoconformity, and tigmothermy.
The mean Tb for R. marina was 25.8 ± 0.5 °C (22.6 - 31.2; n= 17); a similar Tb was exhibited under controlled conditions, at a humidity close to 80%, which could indicate the optimal physiologicalfor this species. Also, its mean Tb is higher than the one recorded within other populations at similar altitudes (600-700 m elevation), but in different geographical areas of South America. Moreover, the mean Tb of this toad is higher than others congeners distributed at altitudes over 1,410 meters. The values of BMR showed trends towards heliothermy, and thermoconformity.
The mean Tb for S. ruber in this study was 23.2 ± 0.6 °C (18.1 - 29.9; n= 37), being reduced than other population at lower elevations, but higher than other congeners distributed over 1200 meters of altitude. These results may suggest that altitude is a determining factor in the Tbs, observing a decline in temperatures with elevation at tropical latitudes. The values BMR showed trends towards tigmothermy and thermoconformity.
The Tbs observed in D. bifurcus, and R. marina were similar contrary to S. ruber which showed a Tb significantly lower. Thus, the thermoconformity tendency of the three species may obey mainly to variation of few degrees between the coldest and the warmest month in tropical latitudes; while the tigmothermy tendency presented for D. bifurcus and S. ruber is more characteristic of shade-dwelling organisms, and finally, heliothermy presented for R. marina seems a strategy to avoid potential impacts of thermal stressor on physiology, ecology, and survival.