1. Introduction
Cocoa (Theobroma cacao L.) is an important botanical species in many developing countries such as Africa, Asia, and Latin America. Cocoa used for chocolate production is treated with a fermentation process for a variable period. During this stage, the microorganisms contribute to the fermentation and consume the nutrients of the mucilaginous pulp surrounding the fresh cocoa bean. Additionally, a series of biochemical reactions occur in the beans due to the increase in temperature, which leads to the formation of aroma and flavor precursors. Subsequently, the fresh cocoa beans are dried, a process that decreases the loss of volatile organic acids and oxidizes polyphenols. The overall result has a great impact on the sensory, physical, and chemical quality of the product (Contreras et al., 2004).
Another treatment of cocoa in the postharvest is the direct sun-drying of unfermented beans. The beans in this type of drying process do not undergo the changes produced by microbial action and temperature increment, and polyphenols are preserved in greater proportion (Chavez-Rivera, 2013; Lippi, 2013). The dried cocoa beans without fermentation are also used for the extraction of cocoa butter with a high yield (Asep et al., 2008).
The pulp surrounding the fresh cocoa beans contains about 80-90 % of water, 10-15 % of sugars (mainly sucrose) and between 0.5 and 0.7 % of proteins. Therefore, the pulp source is the second most important macronutrient (Sarbu and Csutak, 2019). Also, the water activity (a.) of the pulp ranges from 0.98 to 0.99 (Copetti et al., 2011a). These conditions favor the spontaneous growing of fermentation microorganisms, such as lactic acid and acetic acid bacteria, which contribute to the quality of the final product (Mozzi et al., 2013; Copetti et al., 2014). Potentially pathogenic microorganisms can develop too, such as Aspergillus, Penicillium and Fusarium. These molds constitute a risk to public health due to their capacity to produce toxins (Copetti et al., 2011b, Chire et al., 2014). Cocoa can also be a source of chocolate contamination with bacteria such as Salmonella (Nascimento et al., 2013), because of poor hygiene practices during cocoa bean processing (Cordier, 2000; Burndred, 2009). High microbiological levels and the presence of Salmonella are a recognized and reported hazard (Burndred, 2009; Werber et al., 2005). Dried cocoa beans and chocolate have low water activity (a.) (Copetti et al., 2011b) and are high in fat content (Asep et al., 2008), characteristics that contribute to the viability of Salmonella for prolonged periods (Komitopoulou and Peñaloza, 2009). Moreover, it has been reported that a high fat content protects the bacteria in the digestive tract, which reduces the infective dose to less than one colony forming unit per gram (Scott et al., 2009).
Therefore, it is necessary to know the physicochemical properties and microbial group behavior of different types of post-harvest process of cocoa beans.
2. Methodology
Sample Treatment:
Twenty-three cocoa pods with the absence of fissures, bean exposure or fungus growth were selected from a total of cocoa pods during September 2016 (Laboratorio de Salud Pública y Salud Ambiental, Facultad de Medicina Veterinaria, UNMSM). These cocoa pods were cut carefully with a knife to remove fresh cocoa beans by hand. The beans were separated from each other and the average net weight (2120.5 g) of the seeds was registered. Subsequently, half of the weight of cocoa beans were subjected to treatment A and the complementary part to treatment B: Treatment A consisted in the fermentation and drying of the beans in a food dehydrator (artificial drying), while Treatment B only consisted in sun-drying of the beans between 28 to 33 °C. These treatments were performed to obtain cocoa beans used for two different purposes of production: chocolate (Treatment A) and medical products (Treatment B).
Processing of cocoa for chocolate production (Treatment A and A-farmer): Treatment A consisted of two stage: Fresh beans fermentation and then beans drying. For the first stage, a square wooden fermenter box was used (Portillo et al., 2005, Aprotosoaie et al., 2016). This fermenter box has a content capacity of approximately 2 kg (Contreras et al., 2004) and perforations of 0.4 cm on each side and at the bottom. On average, 62 % of fresh cocoa beans was kept in this box for seven days at room temperature (between 20 to 25 °C), covered by a thick cloth to reduce contact with the air. On days 2, 4 and 7, the weight of the bean mass was registered, and temperature was measured with an infrared thermometer. These beans were mixed, so that the lower part could come to the surface, to standardize temperature and aerate the mass (it was carried out in a laboratory in Lima). Treatment A-farmer was carried out as above-mentioned procedure at the cocoa farm in San Ignacio (Cajamarca). The drying stage was performed on day 8, the bean mass was placed in a food dehydrator (Blanik BDA020) at 40 °C (Zahouli et al., 2010). The bean mass total weight was registered at day fourteen.
Processing of cocoa for medicinal products (Treatment B and B-farmer): Treatment B was performed by sun-drying (Aprotosoaie, 2016). The fresh cocoa beans were spread out on a mesh and set in a draft free place. There they received sunlight during seven days at a temperature that was lower than 35 °C (between 28 to 33 °C). The beans were mixed and spread out every two hours from 8:00 to 16:00 every day. At the end of the day the total weight was registered (it was carried out in a laboratory in Lima). Treatment B-farmer was carried out as above procedure at the cocoa farm in San Ignacio Farm (Cajamarca) to verify the quality.
Fresh cocoa beans were subjected to treatments: A, A-farmer, B and B-farmer. To assess results, the following microbiological criteria were used: Peruvian health standard RM No. 591 (MINSA, 2008), and the results of Papalexandratou et al. (2013), Chaves-Lopez et al. (2014), Ardhana and Fleet (2003); and Sangronis et al. (2014).
Analysis methods:
The native cocoa from the province of San Ignacio, located in the department of Cajamarca (Peru) at 1350 meters above sea level, was evaluated using the following methods:
The proximal analysis of cocoa beans was done by triplicate according to AOAC (2016) methods. The analysis included moisture (AOAC 931.04), ash (AOAC 972.15), crude protein (AOAC 970.22), and crude fat (AOAC 920.75ª) (AOAC, 2016). Carbohydrates was determined by difference.
The physicochemical analysis of cacao beans was done by triplicate according to AOAC (2016) methods. The analysis included the determination of water activity (Aqualab series 4TEV DUO instrument, Decagon, USA at 25 ± 0.1 °C) and pH (AOAC 970.21).
The microbiological analysis of cacao beans was done by duplicates according to ICMSF (2000) methods. The analysis included the total aerobic mesophilic count (TAM) employing Plate Count Agar (PCA), mold and yeast count on Potato Dextrose Agar (PDA) and detection of Salmonella in Salmonella-Shigella Agar.
Statistical analysis:
Physicochemical and microbiological analysis were performed by triplicate and duplicate per experimental unit, respectively. Response values were expressed as mean ± standard deviation for each experimental unit. A completely randomized design (p ≤ 0.05) was applied to determine the difference between treatments, using variance analysis (ANOVA). The STATISGRAPHICS 5.0 PLUS® program was used for the statistical analysis.
3. Results
Proximate composition of fresh cocoa beans is shown in Table 1, the amount of water was 56,30 ± 0.02 %. The water content of the beans was like a study that states that the initial moisture content was 55.49 ± 0.14 % (Castro et al., 2016). Treatment A consisted of two processes that had a duration of seven days each one, it had a yield in weight 70.20 ± 11.60 % occurred during fermentation phase and 33.15 ± 2.62 % in dehydrator drying at 40 °C, in this case the conditions were regulated, reached an average moisture of 3.10 ± 0.02 %. Treatment B had a yield in weight of 37.45 ± 11.95 % in sun-drying for seven days and reached 4.81 ± 0.01 % as the average final moisture, this was due to the beans were stirred, spread out and also depends on the environmental conditions (30.84 ± 2.35 °C). It is well known that the yield of weight varies widely due to environmental conditions such as temperature and relative humidity.
Table 1 aw pH and proximate analysis values of cocoa beans
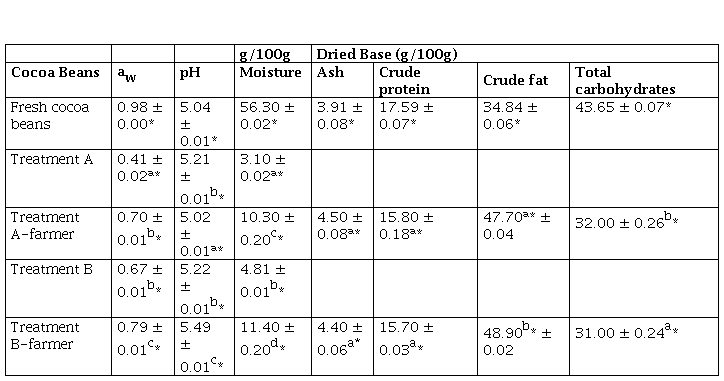
*The values are expressed as mean ± standard deviation n 3
*The values are expressed as mean ± standard deviation (n = 3)
Portillo et al. (2005), indicate that after a bean drying process, the percentage of moisture should be reduced to 6 or 8 %. The moisture content of the cocoa bean after drying is a quality factor that determines a proper packaging, transport, and storage time. These values were like the moisture content reported by Portillo et al. (2005) and were below the limit of 7.5 %, established by Peruvian regulations (MINSA, 2008; INACAL, 2016). Also, Table 1 shows the values of a., pH and proximate analysis of cocoa beans subjected to treatments A, A-farmer, B and B-farmer. It is observed that the fresh cocoa beans had a high a., values that were similar to those found by Copetti et al. (2011a), after each treatment, a. decreased more in treatment A than in treatment B, A-farmer and B-farmer (p ≤ 0.05). pH values of cacao beans increased in treatment A, B and B-farmer (p ≤ 0.05). Crude fat was higher for treatment B than treatment A (p ≤ 0.05), total carbohydrates decreased (p ≤ 0.05). Crude protein and ash were similar (p > 0.05). It was considered only determined full proximal analysis for treatment A-farmer and B-farmer because of significant characterization. Microbiological analysis (Table 2) for all treatment were statistically similar (p > 0.05), the largest reduction in TAM and yeast population were obtained after postharvest, whereas Salmonella was absent in all treatments.
Table 2 Microbiological analysis of cocoa beans
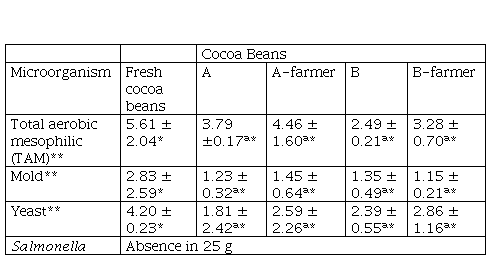
*The values are expressed as mean ± standard deviation n 2 ** results express in log CFUg
*The values are expressed as mean ± standard deviation (n = 2). (**) results express in log CFU/g
4. Discussion
Three important components were analyzed: crude protein, crude fat and total carbohydrate content in fresh cocoa beans. Their respective values on dry base were 17.59 %, 34.84 % and 43.65 % (Table 1). The presence and quantities of these components make cocoa beans a suitable substrate for a wide range of microorganisms, including pathogens, that can obtain energy and nutrients. Furthermore, Gram (-) bacteria, such as Enterobacteriaceae, E. coli and Salmonella, may satisfy more easily their nutritional requirements based on nutrients available in cocoa, compared to Gram (+) which are more demanding (Jay, 2000).
The cocoa farmer prefers to work with Treatment B, because it completes the process in a shorter time (6 to 7 days) and they can obtains dried cocoa beans with and increased of around 2 % higher fat yield (48.90 ± 0.02 %) compared to treatment A (47.70 ± 0.04 %), this value is lower than the one reported by Loo (2020). Both treatments, A and B, experienced weight loss due to release of water, from initial 100 % fresh cocoa beans by weight to 33.15 ± 2.62 % and 37.45 ± 11.95 % dried cocoa beans, respectively. Treatment B was a non-fermentation process, accompanied by the risk that the lack of organic acids would cause the growth of ochratoxin A-producing fungi. It should be pointed out that chocolatiers of fine flavor cocoa require fermented cocoa beans, so the postharvest process takes longer time (14 to 15 days) to ensure the moisture content comply the INACAL (2016) and INEN (2006) Standards.
The cocoa beans of treatments A and B, reached moisture contents of 3.10 ± 0.02 % and 4.80 ± 0.00 %; with a. of 0.41 ± 0.02 and 0.67 ± 0.01, respectively. However, treatments A-farmer and B-farmer performed by the cocoa farmer in the field had higher moisture contents of 10.30 ± 0.20 % and 11.40 ± 0.20 %, respectively (p ≤ 0.05); with higher a. values of 0.70 ± 0.01 and 0.79 ± 0.01 (p ≤ 0.05). It is also known that the final moisture is related to the a. by a sorption isotherm of a food (Belitz et al., 2009). The solar drying depends on the environmental climatic conditions, the heat generated by the solar rays was used to dry the cocoa beans slowly and gradually, which were scattered and removed during the hours of sun, so that the cocoa beans by treatment B-farmer, had a different moisture and a. (p ≤ 0.05) than cocoa beans subjected to artificial drying (treatment A and A-farmer) where the heat was controlled at 40 °C for seven consecutive days, having lower moisture and a.. Furthermore, a. is related to the survival of pathogenic microorganisms, such as Staphylococcus aureus, Clostridium botulinum, Salmonella spp. These microorganisms develop in foods with a. values that range from 0.88 to 0.99. Therefore, the results of the microbiological analysis can be attributed to the low level of a. such as in treatment A. The pH value of the fresh cocoa beans was close to the average acidity due to the pulp that covers the fresh cocoa beans is quite acidic (pH 5.04 ± 0.01). Cocoa beans subjected to treatment B-farmer were higher pH (5.49 ± 0.01) than both treatments B and A; and A-farmer with lower pH (5.02 ± 0.01) because fermentation process produce organic acids (low pH) and drying process release organic acids (higher pH) (Afoakwa et al., 2008; Zambrano et al., 2010). pH also determines the fermentation status, biochemical reactions and tastes of the cocoa beans (Afoakwa et al., 2014), in these terms treatment A and A-farmer were well-fermented cocoa beans. Zambrano et al. (2010) studied the drying process of two cocoa bean types. They did not find marked pH variations (4.6 and 5.1) in dried cocoa beans; however, the pH with treatment B and that of B-farmer was 5.22 and 5.49, respectively, and were statistically different (p ≤ 0.05).
Fresh cocoa beans have the values for TAM and yeast, compared to the four treatments, exceeding 4.00 log CFU/g (Table 2). This is due to the fact that the beans inside the cocoa pod are sterile when it is cut and the beans exposed to the environment, the microorganisms take advantage of the high sugar and moisture content of the fresh cocoa beans to initiate their development (Aprotosoaie et al., 2016; Fowler and Coutel, 2017). According to Peruvian health standard RM No. 591 (MINSA, 2008) states that semi-processed fresh fruits and vegetables, as is the case with cocoa pods that were cut to remove the fresh cocoa beans and before postharvest process, the TAM count must be maximum 4.00 log CFU/g. Papalexandratou et al. (2013), using a culture-dependent technique, carried out the microbial growth of fresh cocoa beans, developed in fermenter boxes from Malaysia and made the following findings: an initial total bacterial population of 6.50 log CFU/g reaching a maximum of 8.90 log CFU/g during the cocoa fermentation process an initial yeast count of 5.30 log CFU/g up to a maximum of 7.00 log CFU/g during fermentation. Chaves-Lopez (2014), reported microbial temporal succession during cocoa fermentation, Ardhana and Fleet (2003) an initial yeast loads of 4.00 log CFU/g reaching a maximum of 8.00 log CFU/g during the cocoa fermentation. TAM and yeast load of fresh cocoa beans of this study was 5.61 ± 2.04 log CFU/g and 4.20 ± 0.23 log CFU/g, values which were close to those found in related studies. Fungal contribution to fermentation is restricted, an initial mold count of 2.00 log CFU/g reaching a maximum of 6.00 log CFU/g were found by Ardhana and Fleet (2003). A mold count of 2.83 ± 2.59 log CFU/g was found in this study (high variation); this might be due to cross-contamination. Salmonella was absence in fresh cocoa bean.
TAM counts were lower in treatment B (2.49 ± 0.21 log CFU/g) and B-farmer (3.28 ± 0.70 log CFU/g), as compared to those that underwent treatment A (3.79 ± 0.17 log CFU/g) and A-farmer (4.46 ± 1.60 log CFU/g), however there were no significant differences between them (p > 0.05). This result can be attributed to the fact that cocoa beans in treatment B and B-farmer were exposed to direct sunlight that may exert a harmful effect on the bacteria (Fonseca and Tabares, 2011). The population of molds was lower in all treatments A, A-farmer, B and B-farmer, and were no significant differences between them (p > 0.05). Peruvian health standard RM No. 591 (MINSA, 2008) does not specify limits for TAM in dried, dehydrated or lyophilized fruits and vegetables. However, for other semi-processed fruits and vegetables, this standard recommends TAM counts below 4.00 log CFU/g. Husk of the cocoa samples for infusions TAM counts were from 4.55 log CFU/g to 3.60 log CFU (Sangronis et al., 2014), the authors stated the Venezuelan standard was a maximum of 4.00 log CFU/g TAM. In this sense, cocoa beans from treatments A, A-farmer, B and B-farmer complied with these criteria. The maximum allowed limit for mold is 2.00 log CFU/g in dried, dehydrated or lyophilized fruits and vegetables (MINSA, 2008). Sangroni et al. (2014) recorded 1.00 log CFU/g mold for husk of the cocoa samples and state the Venezuelan standard was a maximum of 3.00 log CFU/g of mold; and in another study of Colombian fermented food as cocoa beans (Ardhana and Fleet, 2003; Chaves-López et al., 2014) a mold count 4.00 log CFU/g at the end of the postharvest process was determined, therefore, treatment A, A-farmer, B; and B-farmer represented a good manufacturing practices.
In addition, the maximum allowed limit for yeast is 2.00 log CFU/g in dried, dehydrated or lyophilized fruits and vegetables (MINSA, 2008). Sangronis et al. (2014) recorded 1.00 log CFU/g for husk of the cocoa samples and state the Venezuelan standard of 3.00 log CFU/g of yeast; Papalexandratou et al. (2013), found a yeast count of 2.0 - 2.5 log CFU/g at the end of the cocoa fermentation process and Chaves-López et al. (2014) a yeast count of 4.00 log CFU/g by Ardhana and Fleet (2003). Four treatments A, A-farmer, B and B-farmer were no significant different between them (p > 0.05) regarding the microbiological analysis performed. On the other hand, there was not Salmonella presence in any of the treatments. This could be attributed to the fact that this bacterium belongs to the gastrointestinal tract and would be more involved in cross-contamination with gastrointestinal contents due to poor hygiene practices (Nascimento et al., 2013). Since this can occur in the postharvest of cocoa bean sites, some factories wash and dried cocoa beans immediately to ensure microbiological quality before being processed as cocoa derivatives and the subsequent processing of chocolates (Belitz et al., 2009). Finally, due to application of good manufacturing practices, no fungal contamination was found in the cocoa with treatment A, A-farmer, treatment B, and B-farmer in the post-harvest process.
5. Conclusions and recommendations
The physicochemical quality of samples of the native cultivar of cocoa from San Ignacio (Cajamarca, Peru) was evaluated regarding their moisture (56.30 ± 0.02 %), water activity (0.98 ± 0.00), pH (5.04 ± 0.01) and the microbiological count, which complied the national standard. Fresh cocoa beans were processed in four treatments: A, A-farmer, B and B-farmer and the characterization of these cocoa beans in moisture, water activity (a.) and pH resulted statistically different (p ≤ 0.05). The microbiological quality of fresh cocoa beans as raw material should be known. Treatments A, A-farmer, B, and B-farmer had different temperatures; in treatments B and B-farmer, the cocoa was sun-dried at a maximum of 35°C and in treatments A and A-farmer it was artificially dried at 40°C, which showed that the values that are suitable for human consumption (TAM, mold and yeast), including treatment A-farmer with high values in TAM and mold count. Salmonella was absent in all treatments (p > 0.05).
Ongoing microbiological monitoring must also be done in any type of the postharvest to detect the presence of Salmonella and molds. As the cocoa pods heal, the cocoa beans will remain healthy despite the post-harvest process used; thus, the key is to maintain good agricultural practices in plantation.
Acknowledgments: We would like to thank Mr. Angel López and the cacao farmers from San Ignacio in Cajamarca for the materials provided and to Dr. Hassan Firoozmand for scientific communications corrections.















