1. INTRODUCTION
Papaya is a climacteric fruit characterised by a short shelf life time. Therefore, papaya is harvested in a physiological maturity stage that guarantees its commercialisation. A major demand for papaya will keep an upward production trend in the next decade (OECD-FAO, 2015); therefore new post-harvesting techniques or an improvement of the already in use is needed.
Edible films alone or carrying functional compounds, e.g. antimicrobial or antioxidant, have been employed to improve the quality of food (Vargas et al., 2008). The most common polymers on formulation of edible films are proteins, polysaccharides and lipids, which are used alone or combined (Vargas et al., 2008). Starch is an important polysaccharide used in the formulation of biodegradable films (Chiumarelli et al., 2010). Among starches, cassava starch has been widely used on the formulation of edible films thanks to its availability and relative low price (Souza et al., 2012; Chivrac et al., 2010). Bezerra de Aquino et al. (2015) studied the use of cassava starch together with chitosan on guavas whereas Chiumarelli and Hubinger (2012) worked with films based on cassava starch and carnauba wax on apple slices. However no studies of the use of edible films based on cassava starch for papaya (Carica papaya L.) preservation have been developed.
Chitosan is obtained from chitin by deacetylation in an alkaline media (Abdou et al., 2007). Chitosan is a copolymer consisting of β-(1-4)-2-acetamido-D-glucose and β-(1-4)-2amino-D-glucose units. The potential of chitosan as a food preservative of natural origin has been widely reported on basis of in vitro trials as well as through direct application on real complex matrix foods (Durango et al., 2006; Ribeiro et al., 2007). Edible film based on chitosan form a mechanical barrier against fungi (Meng et al., 2008).
Salicylic acid is the 2-hydroxybenzoic acid. It has antimicrobial and antifungal properties (El-Mougy, 2002). Salicylic acid is a resistance inducer and has been tested in fruits and flowers (Yao and Tian, 2005). It is a regulator of vegetal growth involved in the systematic resistance to pathogens (Kessmann et al., 1994) and in the biosynthesis and ethylene action (Raskin, 1992). Therefore, it can be considered an antagonist to ethylene, the main phytoregulator of fruit maturation (Cia et al., 2007). Salicylic acid has been used to alleviate chilling injuries of cucumber and pomegranates (Zhang et al., 2015). However no studies on the effect of salicylic acid on the shelf life of papaya (Carica papaya L.) have been done.
Essential oils are hydrophobic substances with antimicrobial and antioxidant properties. They are normally used as natural preservative in the food industry. The antimicrobial activity is linked to the presence of terpenoids and phenolic compounds (Burt, 2004). Several studies have used essential oils from different sources to inhibit or delay the growth of pathogenic microorganisms (Burt, 2004; Raybaudi et al., 2006). Cinnamon essential oil is recognized as safe (GRAS) for human consumption and is normally used in food formulations, where it fits with desirable sensory characteristics. Cinnamaldehyde is one of the compounds of cinnamon oil and exhibits pronounced antimicrobial properties (Pauli and Schilcher, 2010). Thymol, carvacrol and eugenol are major components in essential oils derived from thyme, oregano and clove bud. They are among the most-studied essential oil compounds (Chinou et al., 2009; Pan et al., 2014) due to their excellent activities against bacteria, viruses, fungi, parasites, and insects (Burt, 2004). The antimicrobial activity of cinnamaldehyde and thymol incorporated into films has been investigated (De Souza et al., 2014; Mastromatteo et al., 2009). However, the use of cinnamaldehyde together with thymol on papaya preservation has not been studied before.
In the present work, preservation of Hawaii papaya was investigated by using edible films based on cassava starch together with salicylic acid or a mixture of cinnamaldehyde and thymol as antimicrobial agents. A comparison between cassava starch films with edible films based on chitosan was also analysed.
2.MATERIALS AND METHODS
Hawaii papaya was bought in El Carmen, Ecuador. Control papayas (uncoated) were washed with water and disinfected with 0,01 % sodium hypochlorite. Afterwards, papayas were immersed in a water solution containing a fungicide (Tiabendazole and Imazalil). Papayas to be coated with edible films were washed with water and disinfected with sodium hypochlorite before applying the film containing solution. Papayas were immersed in the film containing solution and dried at 25 °C. Afterwards, papayas were stored for 28 days at 11 °C and a relative humidity (RH) between 70 and 80 % followed by 4 days at 25 °C and 60 % RH. Analyses were performed each week and 4 days after the storage at 25 °C. Cassava starch (La Pradera, Ecuador) was bought in a local market in Manta, Ecuador. Chitosan, degree of deacetylation
95 % and Mw of 149 kDa, was donated by Universidad Pública de Navarra, Spain.
2.1Film Preparation
Chitosan film was prepared by dissolving 1 % (w/v) of chitosan in 1 % (v/v) acetic acid solution. One percentage of Tween 20, 0,5 % (w/v) glycerol and 0,5 % (w/v) of glucose were added before the solution was homogenised by using an ultraturrax (Polytron, Switzerland) at 11000 rpm for 4 min.
Starch film was prepared by heating a 0,5 % (w/v) cassava starch suspension from room temperature (approx. 25 ºC) up to 90 °C, where it was kept for 5 min. One percentage of Tween 20, 0,5 % (w/v) glycerol and 2 mmol/L of salicylic acid (when it was needed) were added before cooling (Table 1). Glucose (0,5 %, w/v), 0,15 % (w/v) cinnamaldehyde and 0,15 % (w/v) thymol (when they were needed) were added when the solution was at room temperature (Table 1). Afterwards, the solution was homogenised by using an ultraturrax (Polytron, Switzerland) at 11000 rpm for 4 min.
Table 1 Composition of edible film containing solution
| Treatment | CHI 2 (%, w/v) | S 5 (%, w/v) | SA 6 (mmol/L) | C 7 (%, w/v) | T 8 (%, w/v) |
|---|---|---|---|---|---|
| UN1 | - | - | - | - | - |
| CHI2 | 1 | - | - | - | - |
| SSA3 | - | 0,5 | 2 | - | - |
| SCT4 | - | 0,5 | - | 0,15 | 0,15 |
1Uncoated
2Chitosan
3Cassava starch + salicylic acid
4Cassava starch + cinnamaldehyde + thymol
5Cassava starch
6Salicylic acid
7Cinnamaldehyde
8Thymol
A multi-group design with four different treatments was used during the present work. The treatments were uncoated papayas and papayas coated with cassava starch + salicylic acid, cassava starch + cinnamaldehyde + thymol and papayas coated with chitosan. Weight loss, disease severity, texture analyses, soluble solids, titratable acidity and maturity index were measured in papayas stored for 28 days at 11 °C followed by 4 days at 25 °C.
2.2 Weight Loss
Weight loss was calculated by weighing the fruit at day zero and after each storage time. Measurements were performed in triplicate and the results were reported as percentage of weight loss. After weighing, papayas were utilized for the remaining analyses.
2.3 Disease Severity
Disease severity was determined according to Sivakumark et al., (2002). A visual analysis together with a scale from 1 to 5 was utilised, where 1 = 0 % of surface affected area, 2 ≤ 5 %, 3 = 5 - 20 %, 4 = 20 - 50 % and 5 ≥ 50 %.
2.4 Texture Analyses
Puncture tests were performed using the ends and the central part of the fruit. A Shimadzu texturometer (Model EZ-LX, Japan) together with a stainless steel probe of 3 mm diameter and 8 cm length were utilised. The probe was inserted 15 mm into the fruit at a speed of 10 mm/s and the maximum penetration force was recorded.
2.5 Soluble Solids
Half papaya was disintegrated by using a domestic blender. The disintegrated fruit was compressed manually and filtered with a textile. The juice obtained after compression was analysed by using a refractometer (Atago, Japan) according to AOAC (1990). The results were reported as °Brix.
2.7 Maturity Index
Maturity index was determined according to Santamaría et al. (2009). A scale from 1 to 6 was utilised, where 1 corresponded to a green skin with a light yellow stripe; 2: green skin with a well-defined yellow stripe; 3: one or more orange-coloured stripes in the skin; 4: clearly orange-coloured skin with some light green areas; 5: characteristic orange-colour skin of papaya; 6: fruit colour similar to stage 5, but more intense.
3.RESULTS AND DISCUSSION
Figure 1 showed that papayas coated with starch + cinnamaldehyde+thymol (SCT) had the highest weight loss whereas samples coated with starch+ salicylic acid (SSA) had the lowest weight loss along the whole storage time. Statistical differences between SCT and SSA were found on day of storage 7, 14 and 21 (Table 2). No differences on weight loss between uncoated papayas and samples coated with chitosan were found along the whole storage time. Srivastava and Dwivedi (2000) found that salicylates delay the ripening of fruits, probably through inhibition of ethylene biosynthesis, and therefore maintain post-harvest quality. Salicylic acid as an electron donor produces free radicals which prevents normal respiration and therefore leads to low metabolic activity (Wolucka et al., 2005). Low metabolic activity may lead to lower weight loss (Sánchez-González et al., 2011).
Table 2 showed that soluble solids varied between 8,8 and 10,6 °Brix for all samples along the whole storage time. Acidity profile was the same for all samples. It decreased from 0,15 to 0,05 between the first and third week of storage. Afterwards, acidity remained constant up to the fourth week of storage and finally increased again. There was no statistical difference in soluble solids and acidity among coated and uncoated samples. The increase of acidity after the fourth week may be produced by the increase of storage temperature from 11 to 25 ºC. Lechaudel et al. (2010) showed that variations in acid content in mango are due to changes in temperature and time during ripening.
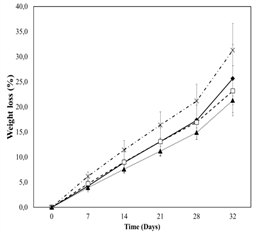
Figure 1.Weight loss of Hawaii papaya coated with edible films and stored for 28 days at 11 °C followed by 4 days at 25 °C. ♦ uncoated, □ chitosan, ▲starch+salicylic acid and ˟ starch+cinnamaldehyde+thymol
Figure 2 showed that penetration force decreased for all samples along the whole storage time. Papayas coated with SCT showed the highest penetration force during the first two weeks of storage, being statistically different than other samples (Table 2). Papayas coated with SSA showed the highest penetration force between the third and fifth week of storage and was statistically different than other samples. The high reduction of penetration force on papayas coated with SCT after the second week could be due to volatilisation of essential oils. Absence of essential oil may lead to changes in fruit texture. Ahmad et al. (2012) observed oregano essential oil evaporation during film formation. Acevedo-Fani et al. (2015) found absence of antibacterial effect of lemongrass essential oil against E. coli due to volatilisation of oil compounds during film formation.
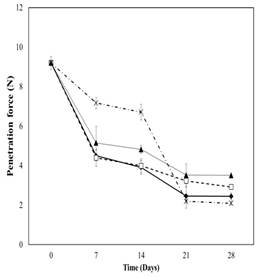
Figure 2 Penetration force of Hawaii papaya coated with edible films and stored for 28 days at 11 °C followed by 4 days at 25 °C. ♦ uncoated, □ chitosan, ▲ starch+salicylic acid and ˟ starch+cinnamaldehyde+thymol
Table 2 Weight loss, soluble solids (ºBrix), acidity, and maximum penetration force of Hawaii papaya coated with edible films and stored for 28 days at 11 °C followed by 4 days at 25 °C.
| Day of storage | Treatment | WL 4 (%) | °Brix | A 5 (mL NaOH 0,01M) | PF 6 force (N) |
|---|---|---|---|---|---|
| 07 | SCT1 | 0 | 9,83a | 0,13a | 9,20a |
| Chitosan | 0 | 9,83a | 0,13a | 9,20a | |
| UN2 | 0 | 9,83a | 0,13a | 9,20a | |
| SSA3 | 0 | 9,83a | 0,13a | 9,20a | |
| 7 7 | SCT1 | 0,06a | 10,07a | 0,15a | 7,17a |
| Chitosan | 0,05a,b | 10,07a | 0,15a | 4,39b | |
| UN2 | 0,04a,b | 9,80a | 0,15a | 4,50b | |
| SSA3 | 0,04b | 9,47a | 0,15a | 5,15b | |
| 14 7 | SCT1 | 0,11a | 10,0a | 0,08a | 6,71a |
| Chitosan | 0,09a,b | 10,13a | 0,08a | 3,99b | |
| UN2 | 0,09a,b | 8,80a | 0,13a | 3,91b | |
| SSA3 | 0,08b | 9,93a | 0,07a | 4,82b | |
| 21 7 | SCT1 | 0,16a | 10,5a | 0,08a | 2,20b |
| Chitosan | 0,13a,b | 10,47a | 0,07a | 3,23a,b | |
| UN2 | 0,13a,b | 10,13a | 0,07a | 2,46a,b | |
| SSA3 | 0,11b | 9,43a | 0,08a | 3,52a | |
| 28 7 | SCT1 | 0,21a | 10,0a | 0,05a | 2,09d |
| Chitosan | 0,17a | 9,47a | 0,05a | 2,92b | |
| UN2 | 0,17a | 10,5a | 0,08a | 2,44c | |
| SSA3 | 0,15a | 10,17a | 0,05a | 3,80a | |
| 32 7 | SCT1 | 0,31a | 10,6a | 0,08a | 1,66c |
| Chitosan | 0,23a | 9,73a | 0,12a | 2,36b | |
| UN2 | 0,26a | 11,0a | 0,12a | 2,38b | |
| SSA3 | 0,21a | 9,67a | 0,08a | 3,12a |
1 Starch + cinnamaldehyde + thymol
2Uncoated
3Starch + salicylic acid
4Weight loss
5Acidity
6Penetration force
7 For a day of storage, same superscript letters showed no statistical difference (p < 0,05)
According to the results of soluble solids and acidity (Table 2) no difference on maturity was found between uncoated samples and papayas coated with different edible films. In fact, results of maturity index (Figure 3) showed no differences among the samples up to the first week of storage. However from the second week, uncoated sample had a higher maturity index compared to coated papayas. No difference in maturity index was observed among papayas coated with chitosan, SSA and SCT along the whole storage time. Delay on fruit ripening was also found by Srivastava and Dwivedi (2000).
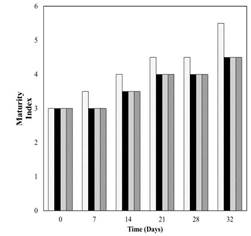
Figure 3 Maturity index of Hawaii papaya coated with edible films and stored for 28 days at 11 °C followed by 4 days at 25 °C. uncoated, chitosan, starch+salicylic acid and starch+cinnamaldehyde+thymol
Treatment of strawberry plants followed by post-harvest treatment of fruits with 2 mmol L-1 salicylic acid effectively controlled the total decay and increased fruit shelf life (Babalar et al., 2007).
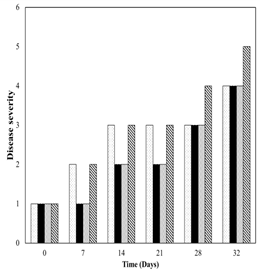
Uncoated samples showed a higher disease severity than samples coated with chitosan or SSA up to the third week of storage (Figure 4). Afterwards, the three samples showed similar results. Increase of storage temperature after the fourth week promoted fungi growth among all samples. Similar disease severity responses of uncoated papayas and samples coated with SCT suggested that physical mixture of starch, cinnamaldehyde and thymol may have a high releasing rate of both compounds during the first week of storage. A high releasing rate of cinnamaldehyde present in starchcinnamaldehyde mixtures was found by Tian et al. (2013). Salicylic acid has been used to control fruit decay on pears during 5 months of cold storage (Asghari et al., 2007) whereas Qin et al. (2003) showed that salicylic acid significantly reduced the incidence of blue mould (P. expansum) and Alternaria rot (A. alternata) in sweet cherry. Regarding chitosan, Ruiz et al. (2010) reported that films based on chitosan and starch reduced the growing of mesophiles (4 Log ufc/g) in fresh cut melon. The antimicrobial mechanism of chitosan was explained by Shahidi et al. (1999) as the action of the positive charges of amino group on the negative charges of the microorganism membrane cells.