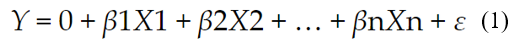I. INTRODUCTION
The lack of food and the global financial crisis led Latin American countries to venture into other livelihood strategies to develop animal production in tropical conditions with higher offers for larger cattle in terms of protein and minerals, which, in general, are in deficit on pastures. In this sense, the biomass of trees and shrubs has an important role due to its considerable levels of protein and acceptable nutritional value. However, due to its benefits for tropical livestock it is necessary to know the essential characteristics of its chemical composition and its impact on acceptability (1).
Together with basic nutrients such as proteins, fats and carbohydrates, plants can produce other compounds such as toxoids, oligosaccharides, flavones, etc. These substances are dispensable molecules for plant metabolism and growth, while their wide variety and diversity of products are key components for plants to interact with the environment in adapting to both biotic and abiotic stress conditions (2).
Thus, it is necessary to know the elements that can constitute a limitation for the use of legumes and non-legumes. For millions of years, many plant species survived thanks to their ability to produce substances that protect them from predators. Even though several of these compounds (condensed tannins, phenols, alkaloids, oligosaccharides and saponins) can produce a violent and immediate reaction, in most cases they have a subtle effect that manifests itself with prolonged ingestion (3).
On the other hand, secondary metabolites are involved in the protection against herbivores, bacteria, fungi, viruses, and even other competing plants. In addition, some plants made use of secondary metabolites as signals for communication between plants and symbiotic microorganisms, as well as served to attract pollinators and seed dispersers (4).
However, these can affect the metabolic processes of the animal and the growth rate of some microorganisms during ingestion (5). According to Isah (4), and Li, Kong, Fu, Sussman and Wu (6), the presence and quantity can vary between species due to biotic factors (primary metabolites products of biochemical and physiological processes of the plant) (7) and abiotic (processes geoclimatic, seasonal changes, ultraviolet radiation, water availability, temperature, soil composition). Processes that, from a physiological point of view, are called elicitors and are molecules or environmental factors that can activate a signaling cascade in plants that mediates the expression of genes related to the biosynthesis of secondary metabolites. They can be classified into two groups according to their origin, as previously mentioned: biotic or abiotic. The former are mainly organic compounds from the cell wall of fungi, yeasts, bacteria or plants. Whereas the abiotic ones are going to be those physical and chemical factors that produce stress in the cells and trigger an enzymatic response, such as UV radiation, drought, salinity, sudden changes in temperature and mechanical damage or the presence of heavy metals (8).
These cause the decrease in photosynthetic activity, and consequently decrease the levels of carbohydrates (glucose, fructose and sucrose) which are mobilized for the production of secondary metabolites. As well as nitrogenous nutrients are also destined for the synthesis of more complex substances from plant secondary metabolism as a defense mechanism, which we call secondary metabolites (7).
Secondary metabolites, in addition to not presenting a defined function in the aforementioned processes, also differ from the primary ones. In that certain groups present a non-uniform distribution in the plant kingdom, that is not all secondary metabolites are found in all groups of floors. They are synthesized in small quantities and not in a general way, often being their production restricted to a certain genus of plants, to a family or even to some species (9).
Gliricidia Sepium (Jacq.) Kunth ex Walp it is classified as a multipurpose tree due to the utilities it presents according to its phenotype, its chemical composition and the edaphoclimatic conditions under which it develops. There is research that evaluates the medicinal properties (10), due to its profile of secondary metabolites, its extracts from different parts of the plant with inhibitory capacity in microbial development, pest control and other diseases for human health and animal species in livestock (11) (12).
If these aspects are considered, the mechanisms and processes through which plant species synthesize secondary compounds and that are caused by the action of external and internal factors and that by different mechanisms the compounds from primary metabolism are used to the production of secondary metabolites. For this reason, it is possible to use mathematical expressions applied to biology as tools to understand this type of phenomenon.
Therefore, it would be important to estimate the content of secondary metabolites in Gliricidia Sepium from the age and content of primary compounds (nitrogen, fructose, glucose, and sucrose).
II. METHODS
A. Research area, climate, and soil
The experiment was carried out in areas of the Teaching-Productive Department belonging to the University of Granma, located in the southeast of Cuba, in the Granma Province, 17.5 km from the City of Bayamo. Studies were carried out for two years (2014-2015) and were considered two periods: rainy (May-October) and dry (November-April).
The soil present in the area was calcic haptustept(13), with a pH of 6.2. The P2O5, K2O and total N content was 2.4; 33.42 and 3 (mg.100g-1 of soil) respectively, with 3.6 % organic matter.
During the rainy season, rainfall was 731.4 mm. The average, minimum and maximum temperature registered values of 26.73; 22.31 and 33.92 ºC, respectively and A relative humidity of 80.78; 51.02 and 96.22 %, for the average, minimum and maximum, respectively. In the period of few rains, rainfall reached values of 270 mm; with a temperature of 24.05; 18.29 and 31.58 ºC for the average, minimum and maximum, respectively, And the minimum, average and maximum relative humidity with averages of 76.21; 44.16 and 97.03 %, respectively.
B. Treatment and experimental design
Was used a randomized block design with four replicates (plots), considering regrowth ages at 60, 120 and 180 days as treatments.
C. Procedures
For the already established species (G. Sepium) at the beginning of each seasonal period, a homogeneity cut was made at 1 m above the ground. The samplings in each plot were carried out taking 10 plants in a row, eliminating the first and last ones to avoid the edge effect in an area of 0.5 ha. The sample was homogenized and weighed, then leaves, petioles and stems were manually separated, the latter with a diameter of less than two cm considered as edible biomass. Then one kilogram was taken for each of the treatments for analysis in the laboratory. No irrigation or fertilization was applied during the experimental stage.
D. Chemical analysis
The samples were dried at room temperature in a dark and ventilated place for 12 days. Subsequently, 300 g were milled for each repetition up to a size of one millimeter. They were stored in amber colored bottles at room temperature until analysis. It was determined: dry matter (DM) and nitrogen (N) by Kjeldhal method according to AOAC(14), while the glucose, fructose, and sucrose contents according to the titration method of Lane and Eynon, which is based on the reduction of copper ions to cuprous oxide by sugar reducing groups in an alkaline medium, using a Fehling solution (cupric sulfate + sodium and potassium double tartrate).
Is necessary a previous sample preparation step for non-reducing sugars, where the glycosidic bonds are broken by hydrolysis, transforming disaccharides and polysaccharides into monosaccharides. Subsequently, these reducing sugars are oxidized in contact with Fehling’s alkaline solution, whose cupric ions are reduced to cuprous oxide, forming sodium salt represented by a brick-red precipitate(14).
E. Validation of models for the estimation of secondary metabolites
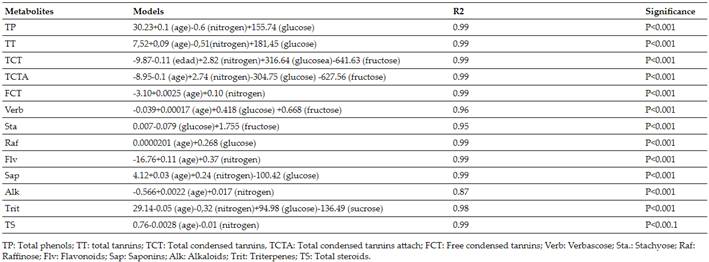
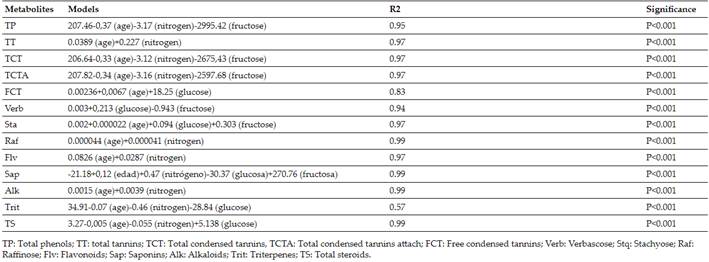
For the validation of the models, those obtained by Verdecia(15)(Tables IandII), as a way of validating the operation of the same, for which it started from the results obtained (of the primary metabolites) at the ages of 60, 120 and 180 days previously described in chemical analysis. These multiple regression equations are shown below where age and nitrogen, glucose, fructose and sucrose are related in both periods of the year:
According to the laboratory results (nitrogen, glucose, fructose and sucrose), and the criteria of Giraldo, Lizcano, Gisjman, Rivera and Franco (16), were considered that the multiple linear regression is a statistical technique that is responsible for analyzing situations that involve more than one variable. This method makes it possible to identify which independent variables are those that can explain an independent variable, verify the causes, and approximately predict the values. Establishing the difference between the observed and estimated values, as well as their relationship. The model prediction is correct when this is set to unity (1) and variability (coefficient of variation) is within the normal ranges.
F. Statistical analysis and calculations
Kolmogorov-Smirnov tests were carried out for the normal distribution of data (17), homogeneity of variances (18), as well as an analysis of variance (ANOVA) of double classification and comparison of means according to Duncan (19). The multiple linear regression model can be described from the following equation (Eq.1):
Where:
Y is a dependent variable
β represents its estimators
ε represents the residual or error
Regression equations (linear, quadratic, cubic, logarithmic and gompertz) were analyzed and the descending method was used to establish the functional relationship between sugars and age. To select the equation with the best fit, were considered the highest value of R², high significance, low error of the terms and estimation, lower mean square of the error, significant contribution of the terms of the equation and low coefficient of indeterminacy (1-R²). For all this, was used statistical program SPSS version 22.
III. RESULT
Table III Effect of Regrowth Age on the Content of Primary Metabolites. abc Values with different letters differ at P<0.05 (19)
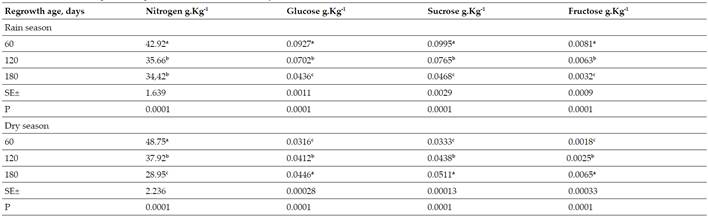
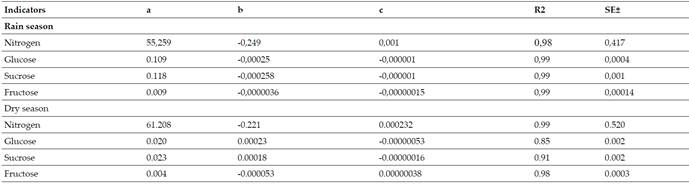
Nitrogen (N), glucose (Glu), sucrose (Suc) and fructose (Frut) contents during the rainy season in G. sepium decreased with regrowth age, with their highest values at 60 days (42.92; 0.0927; 0.0995 and 0.0081 g.kg-1of dry matter). While during the little rain or period of less rainfall the N decreased with age (48.75 g.kg-1at 60 days); Glu, Suc and Frut increased with age, their best values were at 180 days (0.0446, 0.0511 and 0.0065 g.kg-1of dry matter, respectively) (Table III). Quadratic equations were adjusted for all the indicators in both periods, the regression coefficients were higher than 0.98; except for glucose and sucrose during the little rain with 0.80 and 0.91; respectively (Table IV).
Table Vshows the validation results of the models to determine the content of secondary metabolites forGliricidia Sepiumduring the rain, where relationships between estimated and observed values were found. Only stachyose and raffinose presented averages close to 9 and 2 with extremely high coefficients for the first metabolite and 24 for the second, as an average during this period they presented (1.72±2.74 and 11.87); (1.72±2.38 and 13.59) and (1.11±0.219 and 7.81) for the ages under study 60, 120 and 180 days, respectively.
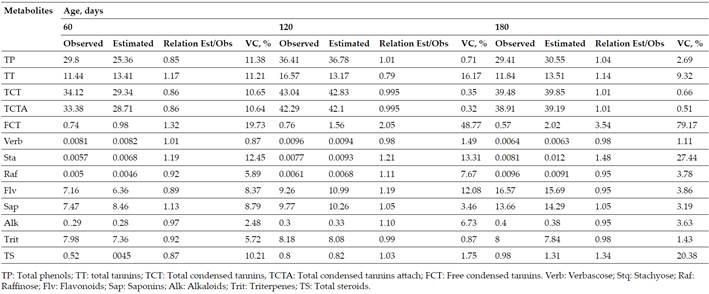
Regarding the validation of models inGliricidia Sepium(Table VI), values were found for the relationship between the estimated and observed, coefficient of variation between (1.00±0.157 and 9.11); (1.12±0.30 and 8.74) and (1.26±0.704 and 12.09) for 60, 120 and 180 days, respectively. Only the free condensed tannins presented averages close to two and variability greater than 19 %.
Secondary metabolites estimated from models forGliricidia Sepiumin the rainy and dry seasons (Table VII). During the rain the TT, FCT, Sta, Raf, Flv, Sap, Alk and TS presented increases with age. While the TP, TCT, TCTA, Verb and Trit did not show a defined behavior with increases up to 120 days and then decreased. On the other hand, in the season of less rainfall, there were increases with age up to 180 days for FT, TT, Flv, Sap, Alk and TS; the TCT, TCTA, FCT, Estq and Raf decreased; and only Verb and Trit showed variability.
Table V. Validation of Multiple Regression Models to Predict the Secondary Metabolites ofGliricidia SepiumDuring the Rainy Season
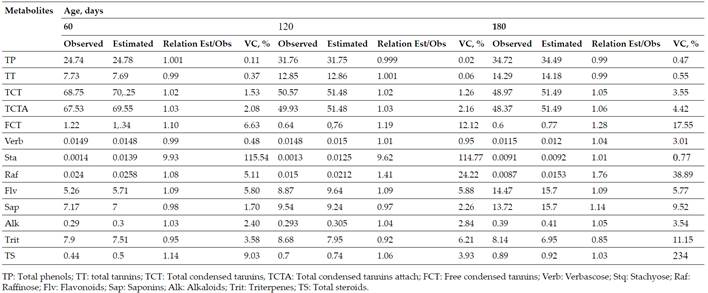
Table VI. Validation of Multiple Regression Models to Predict the Secondary Metabolites ofGliricidia SepiumDuring the dry Season
IV. DISCUSSION
Fodder trees, like other plants, contain numerous organic compounds called primary metabolites, among which are found in the first place, sugars or carbohydrates and nitrogenous substances, which are produced as a result of photosynthesis and are used by plants to many functions, including the synthesis of compounds with greater complexity; Therefore, these primary compounds are important in the interaction of the plant with its environment, and vary according to the maturity of the plant and part of the plant(20).
The determination of secondary metabolites with state-of-the-art equipment and reagents with a certain chemical specification that make their analysis costly and limit the number of samples that can be performed. There is evidence that the production of these is carried out by the plant as a defense mechanism and from the primary metabolites (sugars and nitrogen) which vary according to the regrowth age(1) (2) (3), hence the validity of the models described by Verdecia (15), so that with the use of appropriate mathematical methods, time and resources can be saved to calculate or predict these variables. It is also worth noting that the results achieved on the content of sucrose, glucose, fructose and nitrogen and their variability with age (forage maturity) are within the range described in the international literature for G. Sepium and other shrubby forage plants used in livestock (5) (7) (11) (12) (20).
The decrease in primary metabolites (Table III) was described by Gil-Rodríguez, et al.(5), Jan, et al.,(21), Méndez, et al.,(22), and Paumier, et al.,(23); who inPhaseolus Vulgaris,Moringa Oleifera,Gliricidia Sepiumand medicinal plants reported that the values of carbohydrates and nitrogenous compounds were correlated with the succession of phenological events, from early growth to flowering. From the latter, the general decrease of sugars and protein compounds began, because the behavior of energy metabolites based on morphostructural variations depends on the species, nutritional status and edaphoclimatic conditions in which it is cultivated.
On the other hand, Almario, et al.,(24)reported N values inGliricidia Sepium,Guazuma Ulmifolia,Pithecellobium Dulce,Albizia Guachapele,Acacia FarnesianaandAlbizia Samanof 37.82 and 41.26 g Kg-1DM, respectively, in three cattle-raising regions of Colombia, this reaffirmed that the shrubs under evaluation in this work are within of the normal values for plants of this type.
When evaluating this element Espinosa-Sifontes, et al.,(25)in tropical leguminous treesA. Lebbeck,E. Variegata,E. Berteroana,G. Sepium,P. Saman,D. Cinereareported levels between 37.6 and 44.64 g.Kg-1DM, due to the differences in the edaphoclimatic conditions, management and intrinsic characteristics of each plant.
Milla-Luna, et al.,(26)inGliricidia Sepiumand Ramírez-Pérez, et al.,(20)inTithonia Diversifoliafound that the nitrogen, glucose, sucrose and fructose contents decreased with the regrowth age by 13.15, 0.008, 0.015 and 0.007 g kg-1in the rainy season; while, for the dry season were 17.39, 0.002, 0.003 and 0.0009 g kg-1. This behavior is associated with the fact that the tree's quality varied in the different biomass components. Leaves have higher nutrient concentrations than the branches and stems, and this variation is also related to the age, having the young leaves more nitrogen content than older leaves.
The models obtained and the high R2values reported in this study (Table IV) are similar to those reported by Paumier, et al.,(23), Herrera, et al.,(27) (28), and Verdecia, et al., (29); when determining the effect of age and climatic factors on the nitrogen content in forage species in Cauto Valley. These associated the decrease in nitrogen with the cutting frequency due to the reduction in the synthesis of protein compounds, fewer leaves, an increase in the stem fraction and an increase in the synthesis of structural carbohydrates (cellulose and hemicellulose) and phenolic compounds (lignin).
Ramírez-Pérez, et al.,(20)inTithonia Diversifoliafound quadratic equations were fitted for all the indicators (nitrogen, glucose, fructose and sucrose), with the regression coefficients were higher to 0.95 for all metabolites in both seasonal periods except for sucrose and fructose during the dry season that was 0.93 and 0.91.
Other authors such as Torres-Navarrete, et al.,(30)and Torres-Navarrete, et al.,(31)associated this behavior with the high proportion of stems in the sample, since N values of approximately 33.6 g kg-1DM in the leaves are usually reported in the literature, while in the stems it ranges between 11.2 and 22 g kg-1DM. While Herrera, Verdecia and Ramírez(7), Paumier, et al.,(32)and Estrada-Jiménez, et al.,(33)found nitrogen concentrations of 26-46 g kg-1DM. While Verdecia, et al.,(34)reported similarities in terms of nitrogen content and variability ofT. DiversifoliaandG. Sepium, which emphasizes the importance of using the combination of forage species in animal feed.
In this sense, Hernández-Espinoza, et al.,(35)stated that the stress produced by abiotic factors affects the kinetics of carbohydrate metabolism, so that the balance of environmental factors influences the source-use relationship and, therefore, the final accumulation of these compounds in the storage organs. Hence, in the process of improvement of the different species and selection of genotypes, it is considered that they can withstand the demands caused by adverse abiotic conditions, so that the adjustment capacity or tolerance of plants to these conditions does not be limited.
Thus, the amount of soluble carbohydrates is linked to the morphostructural development of plants. The concentrated reserves of these compounds, to a lesser extent, in the growth points (buds) favor the foliar concentrations of saccharides after the emission of the regrowth. However, although these aspects have been described, from the physiological point of view, the behavior of energetic metabolites depending on the morphological variations depends on the species, nutritional status and edaphoclimatic conditions that the crops have for their development(36).
When referring to the mobilization of carbohydrates, stated that the reserve content achieves its greatest value in the adult leaves at the beginning of flowering, and these are mobilized during the development of the fruit. However, they observed the rapid decrease in the reserves of the roots and leaves during flowering, with a transient increase of these at the beginning of the abscission of the fruits, with a clear decline to a minimum value at the end of the period of physiological abscission. This leads to the nutrition of the fruit, it becomes dependent on photosynthesis rather than on the tree's reserves, aspects that may explain what happened in this investigation(37).
The variations in the concentrations of non-structural carbohydrates ofE. Variegataat the ages of 60 and 120 days reported by Verdecia, Herrera, et al.,(38)are attributed to genetic factors, edaphoclimatic conditions, and the processes to cultivate it. The maturity of the plant, how it is transported and stored, are important when evaluating the carbohydrate content, considering that fructose is synthesized during the early stages of growth. Sugar concentrations in Tithonia were like those reported in the foliage of other non-leguminous plants such asMorus Alba,Trichantera Gigantea,Cnidoscolus AconitifoliusandFicus Carica(39).
On the other hand, according to Verdecia, et al.,(34)there is a close relationship between carbohydrate composition, age and N content in the content of secondary metabolites. According to this study, other factors such as soil and climate conditions can determine the composition of different compounds, particularly α-galactosides. The content ofSaponinsandVerbascosacould be genetically determined, while those ofRaffinoseandStaquiosestem, to a lesser extent, from environmental conditions, and fundamentally from the photosynthetic activity of plants and production of primary metabolites.
Based on the relationship of the secondary compounds with age and the content of their precursor metabolites (nitrogen, glucose, fructose and sucrose) is important to validate the models achieved by Verdecia(15)for the estimation of these. This models allows establishing an accurate and low cost system. Hence, the results on the validation of the models (Tables VandVI) demonstrate the close relationship between the precursor metabolites (nitrogen, glucose, fructose and sucrose) and age, in the secondary metabolites, since only stachyose and raffinose inG. Sepiumin the rainy season; as well as the free condensed tannins inG. Sepiumin the dry season did not show relationships close to one, which makes it difficult, according to Li, Konga, Fub, Sussmand and Wua(40), for the model’s prediction to be perfect.
These behaviors are because the synthesis of each one of these compounds may be limited to specific stages of development of each organism, specialized cells and periods of stress caused by nutrient deficiency or microorganism attack. This phenomenon is due to the phase-dependent formation of the specific enzyme and its activity, which means that the expression of secondary metabolism is based on a differentiation process(4).
Secondary metabolites have a specific and limited distribution in the plant kingdom, they do not appear in all plants, they are synthesized in small quantities, although specific to a certain genus of plants, to a family or species, even influenced by biological stress(41).
Natural substances that originate in plants are synthesized through multiple metabolic routes, and are separated into primary and secondary metabolites, the latter being the most significant relative to their applications in nutrition, animal and human health. The primaries are considered essential or precursors, they are nutritional molecules and can be used in the synthesis of compounds of greater chemical and structural complexity. In this group appear soluble carbohydrates, proteins, lipids and nitrogenous compounds, among others(42).
From collateral pathways to photosynthesis, plants synthesize secondary metabolites(1), these have non-nutritional functions, but are very important for their survival. They are compounds that protect them from external factors, here appear the flavonoids, tannins, lignans, coumarins, alkaloids, terpenes and saponins, among others. These accumulate in various organelles of the cells. For example, flavonoids, which are synthesized in chloroplasts, move to the endoplasmic reticulum and vacuoles.
Unlike other organisms, plants consign a large amount of assimilated carbon and energy to the synthesis of a wide variety of organic molecules that do not seem to have a direct correspondence with photosynthetic and respiratory processes, nutrient utilization, and solute transport, among others(1).
These elements show the reasons why the primary metabolites (nitrogen, glucose, fructose and sucrose) were used in the predictions of the secondary ones. Nitrogen is one of the most important elements in the performance and quality of plants since it is involved in the synthesis of phenolic compounds. Thus, it was evidenced that fertilization decreases these substances(40)especially in early stages of plant growth. This was confirmed inLabisia pumilawhen increasing fertilization from 0 to 270 g kg-1of N ha-1decreased polyphenols(42).
However, the elucidation of the biosynthesis of phenolic compounds will provide the precise dimension of the differences in their content between species and their tissues, and essentially their ecological and evolutionary consequences(43). Although it cannot be ruled out that future knowledge of the genes that encode this process(42)will change or clarify this variety of criteria.
This element (N) is an essential macronutrient, and if its absorption increases, the synthesis of amino acids is accelerated, which are predecessors of nitrogenous compounds such as alkaloids. Therefore, an increase in these with the age of the plant may be due to their protective functions in the plant and nitrogen reserves(44).
The results of the speciesG. Sepiumin both periods of the year (Table VII) agree with those reported by Verdecia(15)who indicated values of 2.71 g kg-1DM of FCT, this allows reaffirming that it is a tropical legume with lower content of condensed tannins, compared toFlemingia Macrophylla,Desmodium Ovalifoluim,Acacia MangiumandCallyandra sp. However, the concentrations are like those reported in soybean meal and in the foliage of other plants consumed without difficulty by cattle under natural conditions(45) (46).
The differences in the amounts of TCT, TCTA and FCT in the present study could be attributed to the differentiation in environmental circumstances, plant nutrition, growth stage, the different reactions of the tannins or other compounds present, and the procedures carried out at the time prepare the samples(3).
However, although the volume and rate of biochemical reactions can increase with temperature, most chemical reactions in plants have a characteristic thermal optimum, which decreases at both higher and lower temperatures. This is due, initially, to the fact that the enzymatic activity and integrity of cell membranes are damaged by extreme temperatures(47).
Different investigations related to the biosynthesis of ET (isoprenoids) point to mevalonic acid as the only predecessor of the hydrophobic skeleton of Saponins. The high positive correspondence between these two groups of secondary metabolites refers to the greater importance of the acetate molecule, compared to that of phosphoenol-pyruvate, in the synthesis of isoprenoids. Hence the low concentrations in which these two compounds appear in the present study(48).
Sánchez, et al.,(49)when evaluating the germination effect of grainsVigna Unguiculata(vigna) var. INIFAT 94,Canavalia Ensiformis(canavalia) var white,Lablab Purpureusvar. Rongai,Glicine Max(soybean) var. IN-CASOY 24 andMucuna Pruriens(mucuna) var. Ash for animal feed, found that the oligosaccharide profile varied with the species under study. The sprouting process increased the total soluble sugars from 20% to 61% and reduced the oligosaccharide content from 98% to 63%.
The results of the estimation of secondary metabolites based on age and predecessor metabolites are like those achieved by Verdecia(15)when grouping different tree, shrub and legume species in the rainy season as well as low rainfall. The varieties evaluated in this research (G. sepium andT. Diversifolia) presented the best results in an integral way with the lowest concentrations of phytochemical compounds.
V. CONCLUSIONS AND RECOMMENDATIONS
The regrowth age had a marked effect on the contents of primary metabolites (N, Glu, Frut and Sac), which explains the close relationship through the established regression equations. Thus, it was evidenced through the validation of the models for the prediction of secondary metabolites, that these in both periods of the year, can be applied due to the dependence of the precursor compounds (nitrogen and sugars).