I. INTRODUCTION
The genus Hylocereus spp. is a cactus that is distributed from Mexico to Central America in the wild, currently in Mexico, there is a planted area of 1 496 hectares. The pitahaya flourishes in areas with temperatures around 30°C and high relative humidity, its flowers are large and white, with a response to photoperiod (1) (2). The flowering is nocturnal, and the floral opening is of a single night, it begins around six in the afternoon and closes the next day in the morning. The flowers are aromatic, up to 46 centimeters long, hermaphroditic with inferior ovary, a single lobe, and a nectarial chamber. The fruit can have a yellow skin or various shades of pink, with white pulp, there are also some with purple skin and various shades of red and red flesh. Worldwide, it is a functional food, which has promoted the increase in the planted area, since the fruit (pulp and peel) is rich in vitamins, minerals, antioxidants and fiber (3) (4). Due to its demand for the food and medicine industry, it is considered an export product, strong internal markets for fresh consumption have been developed in Asian countries such as: Vietnam, Taiwan, Thailand, Indonesia, China (among others) as well as in Israel and Canary Islands; in Latin America: Brazil, Costa Rica and Mexico stand out. Nicaragua is the country that exports 6 160 tons per year.
The fruit of Hylocereus contains several bioactive phytoconstituents such as betalains, which are natural hydrophilic pigments present in most plants of the Caryophyllales order that provide colors ranging from yellow to violet, especially in red pitahaya. Betalains are divided into betaxanthins, which are yellow-orange pigments, and betacyanins, red-violet pigments. (5) (6) point out that such compounds could directly or indirectly provide outstanding biological benefits, making them favorable candidates for the development of innovative drugs. It should be added that the importance of betalains increased due to an increase in the social demand for dyes and natural antioxidants, and products with properties that promote safety and health (5) (7) reported that there is an antiradical potential in the pulp and peel of H. polyrhizus fruits, attributed to its content of polyphenols and ascorbic acid. Polyphenols or phenols, due to effects, have been considered antagonistic to resist free radicals, which deteriorate food and our body (8). High antioxidant activity was found in the ethanolic extract in the peel of H. undatus fruit (8). Various authors such as Omidizadeh et al. (9) , Sudha et al. (8) , and Ibrahim et al. (6) analyzed juice and pulp of Hylocereus with white and red pulp, which is recommended as a promising source of alternative medicine, antioxidant and antidiabetic.
The cultivation of pitahaya shows a problem associated with the origin of planting materials, the type of pollinators and the effect of temperatures. The existing plantations in Mexico are established using materials with high genetic diversity, with free pollination. These materials come from various sources and many are of wild origin, affecting the quality of marketable production. On the other hand, many red-fleshed and red-peel materials are reported as self-incompatible and some white-fleshed materials as partially self-incompatible. (10), require hand pollination to achieve commercial yields under cultivation.
On the other hand, there is a certain level of self-incompatibility in some materials, especially those with red pulp, which indicates that they are incapable of self-pollinating and fertilizing with their own pollen, even if it is viable (11). Another important aspect is that open-pollinated pitahayas in traditional commercial production require animal vectors that transport pollen among individuals with different genetic loads and thus produce fruits with viable seeds. However, low fruit set and lack of pollination are reported due to the absence of efficient pollinators, since the type of pollinator, size and habits affect the amount of pollen that the stigma receives. For example, the flower opens only for one night and bees, with daytime habits, transport a small amount of pollen with their wings or on their legs, compared to the thousands of pitahaya ovules waiting to be fertilized (12).The best pollinators are bats and large nocturnal moths, their habitat has decreased due to the depletion of acahuales and forests, due to changes in land use. On top of this, moths are confused with bats that affect cattle.
Knowledge of the percentages of viable pollen during anthesis can favor conservation, adaptation, and understanding of the physiological behavior of pollen grain fertilization and the most effective time to manually pollinate. Pollen viability is an important variable for genetic improvement programs and is a contributing factor to mitigate aspects of self-incompatibility and fertility that affect the productivity of some species (13). In addition, it is reflected in a better quality of the seed and homogeneity of the fruit. Pollen viability is defined as the ability of pollen to live, germinate and develop, it is a parameter that describes the pollen grain that is capable of germinating both on the stigma, or under artificial conditions, such as in vitro germination, and allows determining the ability of pollen to fulfill its function as a gamete.
Due to all of the above, some producers decide to pollinate manually to ensure fruit set and control the quality of the fruit, the information they access lacks precision regarding the time with the highest amount of viable pollen during anthesis. Knowing the behavior of Hylocereus undatus pollen during anthesis will help the producer to make decisions regarding the use of their available resources. For this reason, it is considered that manual pollination can favor fruit set and improve some quality variables such as size and appearance, it is necessary to provide the producer with information on the percentage of viable pollen throughout the anthesis on schedule, to focus their human and economic resources, where they will have greater security of achieving fruit set, and quality product, which increases its marketable value and does not increase the cost of the crop. Therefore, the research question arises: What is the most efficient time to find the best pollen viability of the flowers, during the anthesis of two cultivars of H. undatus. The research work has the objective of analyzing the viability of pollen during the anthesis of two cultivars of H. undatus with white pulp, taking pollen samples for 13 hours.
II. MATERIAL AND METHODS
The work was carried out in the Cotaxtla Experimental Field (INIFAP) and is located in the municipality of Medellín, Veracruz, Mexico (18° 56' 1.8'' N, 96° 11' 35.5'' W), with an altitude of 10 masl. The average rainfall is 1.336 mm, where the rainiest months are July and September, and the average annual temperature is 26°C. The experiment was with plants from a pitahaya orchard, where two white-fleshed cultivars (H. undatus) were established: called Blanca 1 (B1) and Blanca 2 (B2), the two cultivars had white flesh and pink skin.
In this case, the percentage of viable fresh pollen available in the H. undatus cultivars during the anthesis phase was evaluated. For the above, pollen sample readings were taken every hour, for thirteen hours throughout the anthesis of the flower that happens at night. The technique of staining with tetrazolium salt (2,3,5-triphenyltetrazolium chloride) or TZ was used. This technique has been used for quality control, in stored or fresh seeds, where viability parameters and estimation of vigor stand out (14). The technique was reported by Gaaliche et al. (2013), as an efficient test to determine the rate of viable pollen available. The tetrazolium (TZ) test is a biochemical evaluation (14), where according to what was indicated by the Peters, (2000), seeds are treated with TZ. TZ penetrates cells and reacts with dehydrogenase enzymes involved in cellular respiration in living tissues. As a consequence, the respiratory reactions of the mitochondria of plant cells are catalyzed, during glycolysis and the Krebs cycle. TZ forms a pink/red compound, formazan, which is a water-insoluble, stable and non-diffusible component, so that the reaction with hydrogenase enzymes allows the tissue to acquire a reddish hue (15). This staining makes it possible to differentiate living and therefore viable cells from those that are dead and inviable, due to their respiratory activity, therefore it is a positive indication of seed viability, by detecting respiration at the cellular and tissue level (16) (14). Non-viable tissues do not react or stain, while vigorous tissue stains in colors ranging from pink to carmine. If the tissue is not viable, salt reduction will not occur and the dead tissue will contrast white (not stained) with the viable stained tissue (16) .
Fresh H. undatus pollen was collected from five flowers of two cultivars (B1, B2) during the anthesis of the third floral emission. H. undatus offers up to five emissions per cycle, the second and third being the most abundant. The flowers begin to open at sunset and close the next morning. Pollen was collected every hour from 19:00 to 07:00 the following day, detaching the grains from the anthers (Figure 1A). Pollen collection was stopped at 07:00 am because the flowers began to deteriorate showing signs of wilting and damage to the petals, which indicates that they were either pollinated or senescent without having received pollen.

Fig. 1 (A) Collection of pollen from H. undatus flowers during anthesis. (B) Pollen placed in Eppendorf tubes with tetrazolium salt dilution.
A solution was prepared by mixing 1.0 g of the TZ salt in 100 ml of distilled water, which was diluted to a concentration of 0.25 %. 500 pollen grains were immersed in eppendorf tubes with the dilution (Figure 1B), and they were shaken manually, for a better dilution and contact with the pollen grains (16) (17). In total, 5 H. undatus pollen replicates were considered. The response variable was the color of the pollen grains observed under an optical microscope at 10x, after placing the pollen grains on filter paper. A 2.0 MP CMOS 10x digital microscope camera was used to observe the percentage of viable pollen (obtained by the ratio between the number of viable pollen grains and the total number of each treatment). Viable pollen grains were considered those that presented pink to carmine coloration, which indicates the presence of cell activity in the embryo, while the absence of coloration indicates that there is no presence of cell activity, therefore, it is not viable (16) (17) (18) . The weak staining of the seeds may indicate that they have decreased respiratory activity and less activity of the dehydrogenase enzymes (18). However, they are still viable. The analysis of the variables was through the Kruskall-Wallis statistics (0.05%) and the Pearson correlation coefficient (r) using the XLSTAT program.2016, Addinsoft.
III. RESULTS AND DISCUSSION
The flower of Blanca 2 began anthesis at 18:35, 25 minutes before Blanca 1, which began at 19:00 (Figure 2A); at 21:00 the two materials opened their petals slightly (Figure 2B). The full opening of the flower happened around 10:00 p.m. (Figure 2C).
In both cultivars the decline of the floral structure, wilting and gradual closing of the petals began at 06:30 in the two cultivars (Figure 2D).
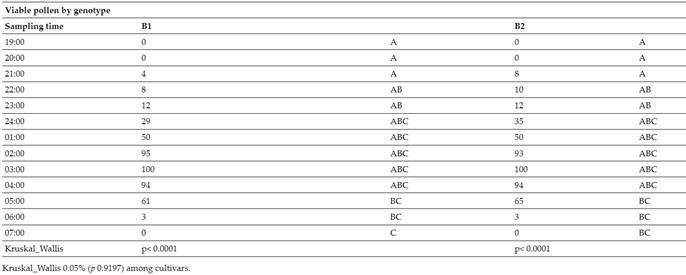
Table 1 shows that from 19:00 to 20:00 no viable pollen was found; from 21:00, the existence of a very low percentage began to be observed. The two cultivars began to show slow growth in the viability percentage from 21:00, which coincides with the semi-opening of the floral structure.
Subsequently, after 24:00 hrs, the increase in the percentage of viable pollen accelerated, reaching maximum viability from 02:00 hrs to 04:00 hrs (02:00-04:00), being 03:00 hrs the maximum point with 100 % viable pollen, and similar for the two materials. At 04:00 hrs, the percentage of viable pollen was reduced and after 05:00 hrs a moderate percentage was observed, but after that time an accelerated drop was observed in the two materials. Sixty minutes later, the viability dropped very abruptly, since it practically disappeared; at 07:00 hrs, no material presented viable pollen, although strong bee activity was observed (Table 1).
Table 1 also indicates a significant statistical difference (p< 0.0001) in the percentages of viable pollen found throughout the anthesis, according to the time the samples were taken for each genotype (B1 and B2) (Table 1). Statistically, it was observed that the viability of pollen in the early morning hours presents a higher probability of finding high percentages of viable pollen, which can be used to collect and pollinate at that very moment. The maximum percentages of viable pollen for the two cultivars were found between 02:00 and 04:00 hrs.
The two materials obtained the same behavior, and there was no statistical difference (p< 0.0001), (Table 1), which may be due to the fact that the two genotypes are H. undatus. However, studies similar to that of Tran et al., (2015), found no significant difference in pollen viability between red and white pulp pitahaya cultivars, nor in germination rates, which is similar to the results of this work.
Figure 3 shows how the staining of the pollen grains was presented in the TZ test, indicating viability (16). In Figure 3A, there are no stained pollen grains, while Figure 3B has 50 %, and Figure 3C indicates 100 %. Figure 3D indicates that the pollen begins to lose viability dropping to 94 % after four in the morning.
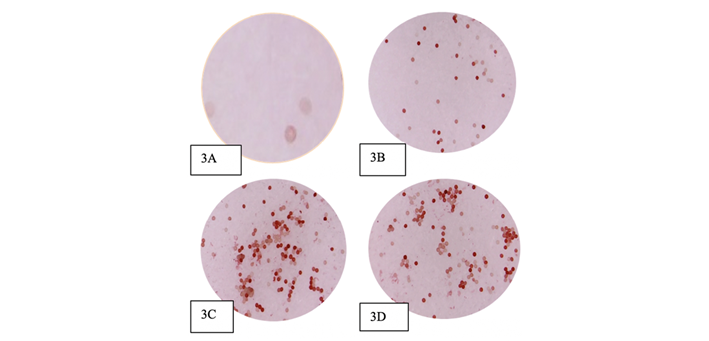
Fig. 3 Pollen seen under the microscope through a computer (3A= 0% from 19:00. to 20:00.; 3B= 50% from 01:00; 3C=95% from 02:00-04:00; 3D=63%, at 05:00)
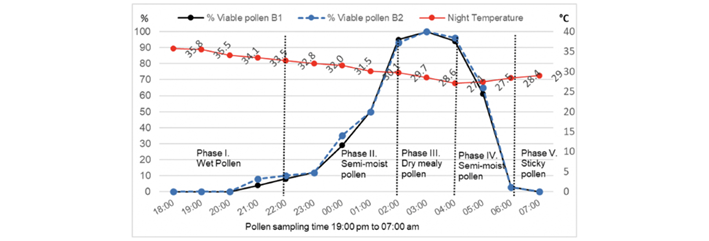
On the other hand, Chu & Chang (19) observed that temperatures higher than 30°C delay the opening time of the flower, as well as its closing, which also affects the viability of the pollen. The data from this study support this statement, since, although the two materials began anthesis from 18:35 and 19:00, the average temperatures recorded were 35.8 and 35.5°C respectively (Figure 4). The total opening of the floral structure occurred until ten o’clock at night (Figure 2C), showing the pollen had a very low viability in the two materials, 8 % (B1) and 10% (B2).
In Figure 4 it can be seen that the viability of the pollen increased as the night temperature decreased, and precisely the materials increased their percentage of viability when the temperature dropped at dawn, which reached 27.5°C (Figure 4). The Pearson correlation coefficients (r), at a significance level of 0.05%, showed negative correlations between night temperature and the percentage of viable pollen observed in the staining, (-0.6677) in B1 and (-0.6721) for B2 (Figure 4).
This also seems to coincide with Zhou et al. (20) who observed that the viability of pollen grains, in their case of American cotton (Gossypium hirsutum L.), are affected by high temperatures.ikewise, Ni et al. (21), found that pollen viability and pollen tube length in pitahayas with white and red pulp decreased in storage at 30°C, so temperature affects both variables. Chu & Chang (19) found that high temperatures affect the viability of pollen and impact the productivity of Hylocereus.
The behavior of the characteristics (consistency) of the pollen was a variable obtained by the researchers through visual and empirical observation based on the experience in the cultivation of pitahaya, and it can be observed in Figure 4, the detachment of the pollen grains from the anthers. When analyzing the characteristics of the pollen, it was possible to differentiate the presence of five phases:
Phase 1, where the pollen is humid and was found to be very adherent to the anthers, with greater difficulty in handling, and where the viability was null or very low.
Phase II, the pollen was found semi-moist and only a partial detachment of the pollen grains from the anthers was achieved, it is the phase where the percentage of pollen begins to increase, with viability in slow growth.
Phase III shows the pollen with a mealy characteristic, very manageable, easily detached from the anthers and it is precisely when the pollen samples observed the highest percentage of viable pollen.
Metz et al. (22) pointed out that for H. undatus and H. Polyrhizus pollen is more humid during the night, reducing in the morning. These data coincide with phases I and II, of the first part of the night, as well as with phase III, which occurs at dawn, which shed floury pollen due to the fact that it is more dehydrated. However, in this work, after phase IV, it was found that the pollen returned to acquire humidity, a low percentage of viable pollen grains, making its handling difficult again. In the latter case, during pollen collection, compact pollen was observed, it became sticky, and according to TZ staining, it lost viability. Therefore, it is suggested that higher moisture content in pollen may be associated with lower viability, similar to what also found Macha et al. (23).
Therefore, the best consistency of the collected pollen, with a high percentage of viable pollen, which favors the success of manual pollination, is from 02:00-04:00. These data are very close to the observations of Ramos (24), although carrying out this activity from phase II is not ruled out (Figure 4). On the other hand, Li et al. (25) found that the greater pollen activity or greater viability does not mean a greater fruit size, since pollinating at 04:00, the fruit set percentage can reach up to 99.2 % but fruit size is significantly 10.5 % smaller.
It is important to add that authors such as Li et al., (25) pointed out that, although the pollen is not yet mature in the Hylocereus pistil, it is possible to apply it to the stigma where it can mature, but an impact of reduction of pollen activity and fruiting percentage will be observed.
IV. CONCLUSION
When studying the anthesis of the flowers of two pitahaya cultivars, it was found that the viability of the pollen collected from 02:00 to 04:00 reached the maximum average viability with an efficient value of 96 %, associated with lower night temperatures. It was observed that the pitahaya flower opens at night and remains open until the next day. It was determined that the viability of pitahaya pollen is 8:00 hrs. Therefore, it can be pollinated from 22:00 hrs at night until 05:00 hrs in the morning. But, in addition, it was found that pollination outside (before and after) the range from 02:00 a.m. to 04:00 a.m. can affect the size of the pitahaya fruit.