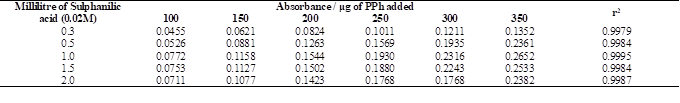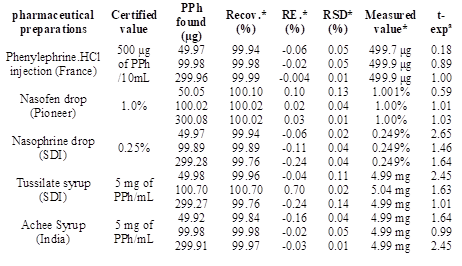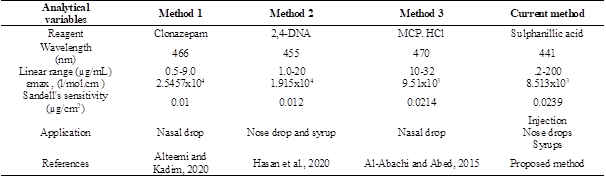INTRODUCTION
Phenylephrine hydrochloride (PPh) is one of the group medicines called sympathomimetics, chemically known as (R)-1-(3-hydroxyphephnyl)-2-methyl amino ethanol hydrochloride. It is a white crystalline powder with the chemical formula C9H13NO2 and molecular weight 203.66 g/mol, used as a nonspecific, decongestant and allergic conjunctivitis, sinusitis and nasopharyngitis, as it works by motivating alpha receptors in areas of specific of the body (British Pharmacopoeia, 2022), It can relieve symptoms but cannot release the cause of the symptoms or speed recovery (Ahmed and Mohammed, 2023).
Several methods were described in the literature for PPh estimation in its pharmaceutical preparations and in the biological fluids. Most of these procedures based on the formation of diazotized PPh and coupling with 2,4-din-itroaniline (Hasan et al., 2020), clonazepam in presence of NH4OH to form a yellow solution measured at 466 nm. (Alteemi and Kadim, 2020), 2-aminothiazole in alkaline medium and followed by dispersive liquid-liquid microextraction method (Mzban et al., 2020), sulfacetamide sodium in alkaline medium of NaOH and the absorbance was measured at 425nm (Wasan, 2019), metoclopramide in an alkaline medium to give an intense orange colored product (λmax at 470) (Al-Abachi and Abed, 2015), p-nitroaniline (Ibraheem, 2009) and 2-aminobenzothiazole (Othman and Abdul Fatah, 2009). Others based on the oxidative coupling reactions of PPh with N,N-dimethyl-p-phenylenediamine in the presence of sodium persulphate (Radhia et al., 2021), N,N-dimethyl-p-phenylenediamine with FeCl3 in alkaline media (Ahmed et al., 2020) and with 4-aminoantipyrine and potassium ferricyanide (Aljeboree and Alshirifi, 2018). Other methods based on the oxidation of PPh either with an excess of bromosuccinamide (NBS) then the residual NBS was estimated by bleaching the color of methyl orange dye in acidic medium of HCl (1mL, 1M) (Zakaria, 2021) nor by adding an excess of chloramine-T and the residual was estimated by bleaching the color of indigo caramine dye (Battu et al., 2018) and PPh was also estimated using spectrophotometric method via flow injection analysis (Al-Abachi and Abed, 2015) and charge transfer complex formation with haematoxylin in alkaline medium (Ahmed and Amin, 2007).
Several analytical techniques have also been published for PPh estimation, these techniques include: RP-HPLC-PDA method (Nagarjuna et al, 2020), high-performance thin layer chromatography (Ragab et al., 2019), chemometric-assisted spectrophotometry and RP-HPLC (Al-Shaalan, 2010), derivative spectrophotometric method (Aljeboree and Alshirifi, 2019), voltammetry (Yagmur, 2018), Conductometric titration method (Hasan et al., 2016). Finally, the dual wavelength method was used for the simultaneous estimated of PPh in bulk and pharmaceutical dosage forms (Suratiya and Pancholi, 2014). However, many of these methods suffer from various limitations, for instance, low stability of the resulting product, low sensitivity and time consuming. Others required solvent extraction, several management steps, temperature control and expensive devices which may not be presented in the laboratory.
In the present work, a very sensitive spectrophotometric method is described for the estimation of PPh drug, which reacts instantly with sulphanilic acid diazotized in an alkaline medium to produce a yellow-colored product with a maximum absorption peak at 441 nm. The method has been successfully applied to determine PPh in some of pharmaceutical preparations.
EXPERIMENTAL
2.1 Apparatuses
A Jasco V-630 digital double beam UV-Vis spectrophotometer equipped with 1.0-cm matched fused silica cells and Bp3001 professional bench top pH meter devices were used for all absorption spectra recording and pH measurements, respectively. Electronic balance ABS 120-4 kern & Sohn Gmbav, was also used for weighing chemical materials.
2.2 Reagent and chemical solutions
High pure chemical materials were used in all experiments.
Standard PPh solution (100 ppm): A 0.01 g of PPh substance obtained from the (SDI) company (for drug industries and medical appliances, Samarra-Iraq) was dissolved in a quantity of distilled water (DW). The solution was transferred to a 100 mL standard flask and the volume was then completed to the mark using DW.
Sulphanilic acid solution (0.02 M): A 0.3464 g of sulphanilic acid was dissolved in 100 mL of DW using standard flask.
Sodium nitrite (0.02M): A 0.1380 g of sodium nitrite (Analar), was weighted and dissolved in a quantity of DW and the volume was then finalized to 100 mL with the same solvent.
Hydrochloric acid (0.1 M): A 0.86 mL of (11.6 M) concentrated hydrochloric acid (Honey well) was diluted to 100 ml using DW.
Sodium carbonate solution (1 M): A 10.5988 g of sodium carbonate was weighed and dissolved in 20 mL of DW. The solution was placed in a standard flask of 100 mL and then diluted with DW.
2.3 Essential procedure
To a series of 25 mL standard flasks, a 1mL of sulphanillic acid, 1 mL of 0.02 M of sodium nitrite and 0.5 mL of 0.1 M hydrochloric acid were added to each flask and mixed well. After waiting for 10 minutes, an aliquot of PPh containing 0.2-20 μg ml-1 and 1 mL of 0.5 M sodium carbonate solution were added. The contents of the flasks were diluted to the mark with DW and the absorbance of the sample was recorded at the selected wavelength 441 nm versus blank solution.
2.4 Analysis of PPh in the pharmaceutical preparations
2.4.1 Nose drops analysis
-Nasofen (1%) solution: A 1.0 mL of nasofen drop (1%) (pioneer company) was taken and placed in a standard flask of 100 ml, then the volume was supplemented to the mark with DW to obtain a solution of drug containing 100 µg/mL of PPh.
-Nasophrine (100g/mL) solution: A 0.25 mL of nasophrine drop (0.25%) (obtained from SDI, Samarra-Iraq) was withdrawn and placed in a 100 mL standard flask, the volume was then supplemented to the mark by adding DW to obtain a solution of the nasophrine drop containing 100 µg/mL of PPh.
2.4.2 Syrups analysis
-Tussilate syrup (2.5 mg/5 mL) solution: A 5.0 mL of the tussilate syrup (SDI, Samarra-Iraq) was diluted to 50 mL with DW using standard flask to obtain a solution containing PPh its concentration 50 µg/mL (Hasan et al., 2020).
-Achee syrup (2.5 mg/5 mL) solution: A 5.0 mL of the syrup solution (Brawn-Indian) was quantitatively transferred to a 50 mL standard flask and diluted with DW to the mark to get a solution containing 50 µg PPH /mL.
2.4.3 PPh injection (500 µg/10 mL): Each ml the injection containing 100 µg of PPh /mL
An aliquot of the diluted drug solutions prepared (nasofen, nasophrine, tussilate and achee syrups and PPh injection) was then treated as the recommended procedure.
RESULTS AND DISCUSSION
The effect of various analytical variables on the color development and the stability of the azo dye produced has been studied and optimized.
3.1 Principle of the method
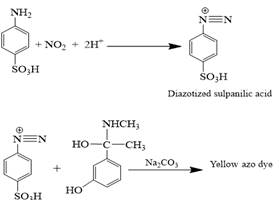
The principle of the proposed method depends on the reaction of sulphanilic acid with an equivalent amount of sodium nitrite in an acidic medium to form diazonium salt, which then coupled with PPh to produce a water soluble yellow azo dye gave maximum absorption at 441nm, as shown in the following equation (Figure 1).
3.2 Effect of acid type on absorbance
The influence of several amounts 0.3 - 3.0 mL of various strong and weak acid solutions (0.1M) have been studied on the absorbance of azo dye. The results are shown in Table 1.
Table 1 Effect of various acids (1M) on absorbance of azo dye by using [300 μg of PPh + sulphanilic acid(1mL) + NaNO2(1mL) + NaOH (2mL)]
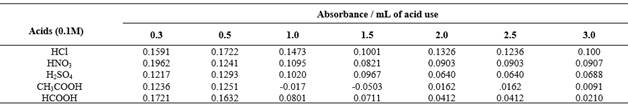
The results in Table 1 reveal that 0.5 mL of hydrochloric acid at a concentration of 0.1 M gave the best absorbance value, so it was adopted for the next experiments.
3.3 Reaction time effect
The influences of diazotized reaction time on the absorption intensity of the azo dye was carried out by measuring the absorption at different periods of time at laboratory temperature (25±2C⁰).
3.4 Influence of sulphanilic acid amount
The influence of different amounts of sulphanilic acid as a diazotized agent on the absorption intensity of azo dye was carried out using 0.3-2.0 mL of 0.02 M sulphanilic acid. The data are summarized in Table 2 and indicated that 1.0 mL of 0.02M sulphanilic acid gave the high sensitivity (i.e. the highest absorbance value) of the resulted azo-dye and perfect estimated coefficient (r2=0.9995), so it was chosen for the following experiments.
3.5 Effect of base type and its amount
The influence of several quantities 0.3-2.5 mL of various bases (sodium hydroxide, potassium hydroxide, sodium carbonate and sodium bicarbonate) on the absorbance of azo dye has been studied and the results obtained revealed that the volume of 1ml of sodium carbonate solution (1 M) is the optimum because, it gives the best sensitivity (the highest absorption value), therefore, it has been adopted for the subsequent experiments.
Time effect on the color development
The resulting yellow-colored azo dye appears immediately after adding the alkaline solution of sodium carbonate and remains stable for at least 90 minutes.
3.6 Order additions effect
The effect of different sequences of additions of the reaction components on the absorption intensity of azo dye was also examined. The experimental results shown in Table 3 revealed that the sequence of reactants according this order (sulphanilic acid + NaNO2 + HCl + PPh + Na2CO3) at 441 nm was the optimum.
3.7 Absorption Spectra
Under the ideal conditions of the procedure, a yellow azo dye was formed by coupling of PPh. with diazotized sulphanilic acid in alkaline solution of Na2CO3. The resulting azo-dye displays maximum absorption at 441 nm versus the solution of blank which show a negligible absorption at this wavelength. Figure 2 shows the final absorption spectra.
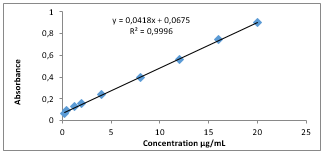
3.8 Reproducibility and Validity of Beer's law
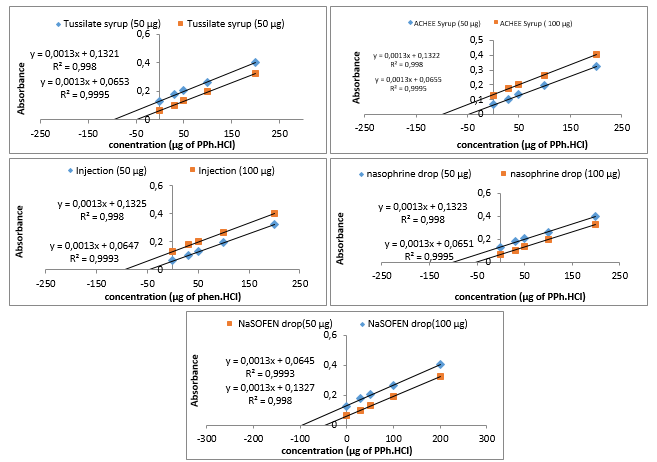
A linear calibration graph was attained by plotting the absorbance against concentration of PPh which was compatible
Beer's law within the concentration range of 5 to 500 µg of PPh in a final volume 25 mL (i.e. 0.2-20 µg/mL) with estimated coefficient (r2=0.9996) As shown in Figure 3.
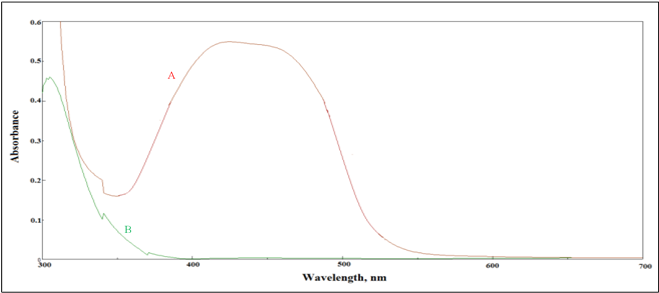
Figure 2 Absorption spectra of 4 µg/mL PPh treated according to the recommended procedure and measured Vs. (A) blank, (B) blank measured Vs. distilled water
The molar absorptivity was estimated and equal to 8.51x103 L/mol.cm, which corresponds to Sandell's sensitivity of 0.0239 µg/cm2. The detection and quantification limits (LOD) and (LOQ) were measured and established to be 0.0050 and 0.0166 µg/mL, respectively (International Conference on Harmonization, 2005). A relative error percentage and the relative standard deviation were calculated and set up to be in the range -0.374% to -0.147% and ±0.037 to ±0.28, respectively. The stability constant (Ks) of the resulting product was also estimated (Azeez and Mohammed, 2022) and was equal to 5.7984106× L/mol (Hargis, 1988).
3.9 Azo dye stoichiometry
Under the optimized conditions of the essential procedure, Job’s and molar ratio methods (Delevic, 1997) were used to clarify the correlation ratio of PPh with the diazotized sulphanilic acid (SA). Both methods were carried out by using the same concentrations of PPh and SA solutions (4.91×10-4 M). The results of both methods are explained in Figure 4 reveal that the azo dye was formed by the reaction of PPh and diazotized SA in a ratio of 1:1.
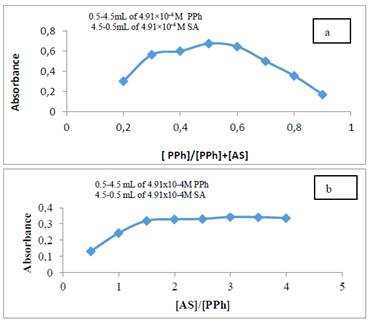
Figure 4 (a) Job and (b) molar-ratio plots for PPh- diazotized SA [PPh]= concentration of phenylephrine, [AS]= concentration of reagent
According to the results of stoichiometry study the stoichiometry of the resulting azo dye which formed through the diazotization and coupling reaction of PPh and salphanilic acid can be clarified as Figure 5.
3.10. Application of the method
The proposed method has been used for the analysis of PPh in its pharmaceutical forms (injection, drops, and syrups) from different origins and at three different quantities (50, 100, 300 μg). The results are listed in Table 4, it indicates that the proposed method is a suitable for determining PPh with an acceptable result.
To evaluate the results of the proposed method, a t-test has been investigated. The results in Table 2 reveal that the values of t-exp. are less than the t-tabulated value (2.776) at the 95% confidence level and for four degrees of freedom (N=4) (Gary, 2004). The results indicated that the difference is statistically not significant, which confirms the success of applying the proposed method to estimate PPh in its drugs.
3.11. Standard additions method
In order to evaluate the selectivity of the proposed method and its success in estimating PPh in its pharmaceutical preparations, the standard additions method has been applied. The results are exemplified in Figure 6 and listed in Table 5, they indicate that the standard additions method agrees well with the results of the proposed method within an satisfactory range of error.
* Average of five estimations, a t-exp.: t-experimental,
Table 5 Analysis of phenylephrine hydrochloride in pharmaceutical preparations by standard additions method
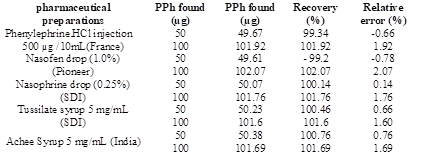
Comparison with other methods
Table 5 shows the comparison between some of analytical variables for the suggested method with that of another literature spectrophotometric method, which utilized the same procedure (forming color azo-dye).
CONCLUSION
This work describes a simple and sensitive spectrophotometric method for the estimation of PPh in its pure form and in certain drugs through diazotization and coupling reaction. The method does not require solvent extraction steps or temperature control. The method is also precise and accurate to be successfully applied for estimating PPh in injection, nose drops and syrups.