1.INTRODUCTION
Synthetic adhesives derivate from petroleum resources are used in countless applications, due to their versatile mechanical, physical and chemical properties (Dinte and Sylvester, 2018). As a result of the excessive dependence on fossil-raw-materials and environmental concern; greater efforts have focused on the development of bio-based alternatives than traditional adhesives (Heinrich, 2019; Richter, et al., 2018).
Polyvinyl alcohol (PVA) is a thermoplastic synthetic biodegradable polymer. Its physicochemical characteristics and low cost had expanded the use of PVA in food, packaging, textile, manufacturing, paper and paint industries as well as in medical and pharmaceutical applications (Demerlis and Schoneker, 2003; Limpan, et al, 2012). Due to its nontoxic nature and water solubility; PVA becomes a potential alternative to develop bio adhesives (Maria, et al., 2008).
Over the last decades numerous bio-based adhesives have developed with a number of different renewable raw materials such as proteins, polysaccharides, polyphenols and lipids (Ferdosian, et al., 2017; Heinrich, 2019; Pizzi and Mittal, 2017). Starch, a naturally occurring hydrophilic polysaccharide, is formed by amylose and amylopectin; though, the ratio of these components vary depending on edaphoclimatic factors (Ferdosian et al., 2017; Zhang et al., 2015). Starch is currently used in adhesives, carpets, textiles, paper and water treatments as well as in medical applications as excipient, absorbent, and encapsulant (Kavlani, Sharma and Lalit, 2012; Menzel et al., 2017).
From the perspective of adhesive formulation, native starch shows some limitations in terms of bonding capacity, thermal decomposition and storage stability. Therefore, the modification of starch microstructure is highly required. Particularly, modified starches increase the solids content leading to shorter drying times and higher bond strength (Qiao et al., 2017). Furthermore, it has been reported that some modification procedures reduce retrogradation phenomena (Ashogbon, 2018).
Physical, chemical, enzymatic, and genetic modifications are commonly used for starch derivatization (Gadhave, et al., 2017; Kaur, et al., 2012). Nevertheless, chemical modification is one of the most versatile route due to starch molecule separation into small units and potential reactivity of hydroxyl groups (Bhattacharyya, et al., 2015; Kavlani et al., 2012). Furthermore, chemical derivatization has been combined with other modification methods such as extrusion and microwave to enhance starch properties (Kaur et al., 2012; Kavlani et al., 2012).
Urea derivatization, a well-known chemical modification method, has widely been used to carbamate formation (Sun, Gu, Tan, Zhang, and Huo, 2018). Moreover, starch carbamate is produced as result of the reaction of the starch with urea in solid state at high temperatures. In this context, it has been proposed that starch carbamate improve bonding capacity of adhesives (Menzel et al., 2017; Tomasik, 2003).
Additionally, several investigations have focused on starch modification through emerging technologies like high hydrostatic pressure, ultrasound, electrical pulses and radiation. These technologies are environmentally friendly and less polluting, than traditional techniques. Specifically, radiation has shown significant benefits in chemical modification of starches (Ashogbon, 2018). Low levels of γ-radiation promote the formation of free radicals which in turn generate not only solubility increase but also reduction of viscosity and swelling power (Atrous et al., 2015; Sokhey and Hanna, 1993). However, doses greater than 100 kGy have shown to damage the structure of the polysaccharide (Sokhey and Hanna, 1993).
In recent years, several starches namely, wheat, corn, rice, cassava and potato have been studied in eco-adhesives elaboration (Gadhave et al., 2017; Turunen et al., 2003). However, limited number of investigations focused on non-traditional Andean starches have been reported in specialized literature. In order to contribute with alternatives to reduce non-renewable resources dependence, the aim of this research is to study single chemical modification and dual modification (gamma irradiation and chemical modification with urea) of achira (Canna edulis) starch to develop bio-based adhesives.
2. METHODOLOGY
2.1. Materials
PVA (88 % hydrolyzed) was procured from Sekisui Chemical Co., Ltd. (Japan). Food grade achira starch (23,8 % amylose) was purchased from Colinas Verdes Foundation (Ecuador). All reagents (urea, sulfuric acid, ethanol, glycerol, sodium carbonate and hydrochloric acid) used were of analytical grade. Plywood veneers were obtained from Endesa-Botrosa (Ecuador) and cut into strips (100 mm x 15mm). The thickness of veneers was 3 mm.
2.2. Methods
The starch used in this research was modified in two stages:
-Electron Beam Processing
Native achira starch was dried at 40 °C for 24 h and packed up in polyethylene bags (thickness of 49 mm, area of 70 mm x 80 mm). Then it was treated with a scanned electron beam using a linear accelerator ELU-6U with 6,8 MeV mean energy and mean dose rate of 19,5 kGy/min at ambient temperature and normal pressure. The irradiation dose (50 kGy) was checked using cellulose triacetate film.
-Chemical modification of starch
For the production of starch carbamate, irradiated starch was initially hydrolyzed, following the methodology proposed by Mostafa, (2003). Starch and 1 N HCl (1:5) were mixed at 50 °C for 60 min and neutralized with 14% sodium carbonate solution. Ethanol was added to precipitate the hydrolyzed starch. The product was dried in an oven at 60°C for 2 h.
Starch carbamate was prepared according the method proposed by Menzel et al., (2017). Urea (20 % of the amount of starch), 1 mL sulfuric acid and 15 mL ethanol were blended with hydrolyzed starch. The mix was heated in an oven at 100 °C for 3 h. The product was washed in ethanol:water (70:30) and centrifuged at 1000g for 10 min.
Characterization of modified starch:
- FTIR
Infrared analysis of starch was recorded on a spectrometer Perkin Elmer, model Spectrum One. Data were collected over 16 scans with a resolution of 4 cm-1 in a range of wavenumber from 4000 to 650 cm-1.
-Water binding capacity
Water binding capacity was measured according to Yousif, et al., (2012). A gram of starch and 15 mL distilled water were centrifuged at 1250g for 20 min. The supernatant was discarded, and the weight of water bound by the dried sample was measured.
Preparation of adhesives
Water, glycerol, ethanol, and mineral oil were stirred for three minutes at 200 rpm. PVA was added to unheated solution in ratio 1:5 under continuous agitation and the suspension was heated at 85 °C and stirred for 30 minutes at 75 rpm. The solution composition is presented in Table 1.
Table 1 Composition of PVA solution
| Component | Percentage on the PVA solution (%) |
|---|---|
| PVA | 20,00 |
| Water | 68,00 |
| Glycerol | 10,00 |
| Ethanol | 20,00 |
| Mineral oil | 2,00 |
Once the PVA solution was obtained; starch, 0,2 g of sodium benzoate and five grams of water for each gram of starch were added to the solution with continuous stirring. The final composition of each adhesive, with PVA composition constant, is described in Table 2.
Table 2 Starch-PVA adhesives composition
| Sample | Starch | Composition (g starch/g PVA solution) |
|---|---|---|
| M1 | Native | 0,06 |
| M2 | 0,17 | |
| M3 | 0,28 | |
| M4 | Modified | 0,06 |
| M5 | 0,17 | |
| M6 | 0,28 | |
| M7 | Dual-modified | 0,06 |
| M8 | 0,17 | |
| M9 | 0,28 |
Adhesive characterization
-Shear bond strength test
Shear bond strength was performed according to the methodology proposed by Kim and Netravali, (2013) using a universal testing machine Instron, model 3365. Plywood strips were sanded with a 600 grit-sandpaper. Two plywood strips were settled as shown in Figure 1; adhesive was applied on a 15 mm x 15mm area at the end of the strips with a multiple clearance square applicator (5 mils). The crosshead speed was 1mm/min.
Shear bond strength τ was calculated by equation (1), where Pm is the maximum load at break and A is the joint overlap area. Five specimens were tested for each sample.
-Determination of apparent viscosity
Apparent viscosity of adhesives was determined with rotational viscometer Fungilab, model EVO Expert at 4 rpm using spindle number TL9. The viscosity was measured in triplicate at 25 ± 1 °C.
3. RESULTS AND DISCUSSION
3.1 FTIR evaluation
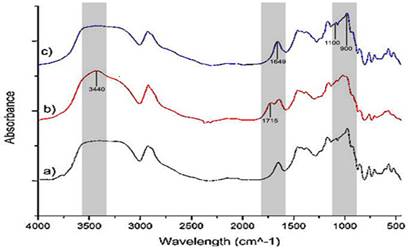
FTIR results are presented in Figure 2. On the whole, infrared spectrums showed a broad band in the range of 3600-3000 cm-1 corresponding to OH stretching vibration and CH stretching functional groups (Kizil, et al., 2002). A band at 3440 cm-1, ascribed to NH asymmetric stretching of carbamate group, was identified in modified starch spectrum (Menzel et al., 2017). Moreover, the band at 1715 cm-1 corresponding to carbonyl (C=O) stretching vibrations ratified starch carbamate formation. (Siemion, et al., 2004).
Dual-modified starch did not present NH and C=O bands, suggesting electron beam action inhibited the formation of starch carbamate. This behavior is probably associated to the higher degradation caused by radiation in comparison with the chemical route (Wu and Song, 2006). According to Wang, et al., (2017) the absence of C=O band could be a result of high degree of deterioration of active sites in starch.
Furthermore, the variation of the band intensity located at 1649 cm-1 was observed. This corresponds to the water absorbed in the amorphous starch region (Kizil et al., 2002). As a result, greater amount of water could be absorbed (Menzel et al., 2017). The destruction of the crystalline structure of the starch by the electron beam is greater than the action caused by chemical substances, since the radiation achieves a greater penetration into polysaccharide structure (Wu and Song, 2006). Otherwise, Warren, et al., (2015), reported that modification treatments decrease intensity of the bands associated with C-O and C-C stretching vibrations and C-O-H bending from glycosidic bonds.
3.2 Water binding capacity
Table 3 shows the binding capacity of native and modified starches. It is noticed that global modification treatment affected hydrogen bonds. The results suggest that high polarity of starch carbamates had an important influence in water binding capacity (Darmanin and Guittard, 2015). In addition, hydrogen bond formation occurs preferentially between starch carbamate and nearby starch molecules (Menzel et al., 2017; Wilpiszewska and Spychaj, 2007).
Table 3 Water binding capacity of native and modified starches
| Sample | Water binding capacity (g/g) |
|---|---|
| Native starch | 0,91 ± 0,02 |
| Modified starch | 1,26 ± 0,13 |
| Dual-modified starch | 1,18 ± 0,09 |
On the other hand, gamma irradiation doses contribute to the acidity and solubility in water as well as to the decrease of viscosity in starches. The enhanced solubility was produced due to the division of the chain under irradiation and consequently decrease of hydrogen bonds between chains and the formation of smaller molecules such as sugars and dextrins (Mathias, et al., 2016). Despite this, the reason for the lower value (1,18 ± 0,09) for irradiated starches, in comparison with chemically modified starches (1,26 ± 0,13), is due to the absence of C=O carbamate group.
Starch molecules experienced significant changes in their structure due to the degradation phenomenon induced by ionizing radiation processing (Liu, Ma, Xue and Shi, 2012). According to Bhat and Karim, (2009), the application of ionizing radiation generates free radicals which are able to promote molecular changes and fragmentation of starch. This property has been suggested to be one of the main mechanisms underlying physicochemical changes in starch, like reduction of viscosity and high-water solubility.
3.3 Characterization of adhesives
-Shear bond strength test
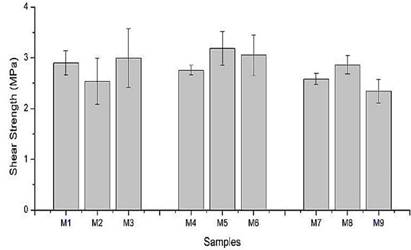
Figure 3 shows shear bond strength results. Overall, no significant differences among samples were observed. However, it is noticed that shear bond strength of dual modified starch (M7, M8 and M9) was slightly lower than the other samples. This characteristic is related not only to the number of polar functional groups (Mathias et al., 2016) but also with the molecular weight decrease due to radiation process (Kamal et al., 2007). Besides, the high polarity of C=O groups allowed to improve adhesion on lignocellulosic substrates (Mathias et al., 2016; Neuman, 2004).
Imam et al., (1999) reported that increase of starch concentration leads to a better interaction between starch and cellulose derivatives of the wood. However, this trend was not identified in the presented study which is possibly ascribed to non-controlled adhesive thickness applied in plywood (Mathias et al., 2016). Indeed, viscosity variation of tested samples leads to heterogeneous thickness applied and consequently changes in shear bond strength.
-Apparent viscosity
Viscosity is a determining factor in adhesives production, since it allows to control its wettability on substrates. Adhesives with high viscosity cannot spread appropriately on adherent surface, and consequently have a low bond strength. On the other hand, adhesives with low viscosity flow easily, spill from the surface, and do not achieve good adhesion (Wang et al., 2017).
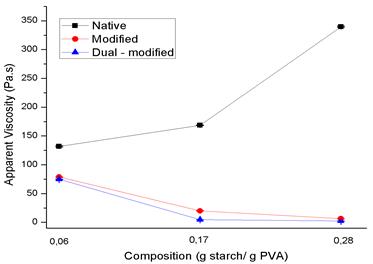
Results of apparent viscosity are presented in Figure 4. Noticed that the viscosity of native starch adhesives exhibits an increasing trend as a function of the increment of starch-PVA ratio.
The gelatinization process is associated to the starch concentration due to the leaching of amylose that occurs when the starch granules are heated, swell and break their structure (Menzel et al., 2017). The viscosity increases due to the greater interaction between the hydrogen bonds of the polysaccharide molecules with those of water (Lewicka, Siemion and Kurcok, 2015).
Contrary to the results of native starch samples, the viscosity of the modified and dual-modified starch adhesives decreases as the starch-PVA ratio increases. It should be noted that, in both cases, the starch used in the adhesives was subjected to a process of depolymerization by acid attack, which causes the polysaccharide chains to break (Ashogbon and Akintayo, 2014). Moreover, with the dual-modified starch, there was a previous process of depolymerization by the attack of the electron beam, which increased this degradation (Lee et al., 2006).
Kamal et al., (2007) points out that starches exposed to modifications by chain decomposition exhibit low viscosities due to the breakdown of glycosidic bonds between glucose molecules. Once these bonds are cut, they cannot be joined again with the same force and the viscosity of the modified starches is always lower than that of the native ones (Kaur et al., 2012).
4. CONCLUSIONS
This research studied two methods of starch modification and their effect on the shear bond strength and apparent viscosity. The single modified starch showed two characteristic bands at 3440 cm-1 and 1715 cm-1 corresponding to NH stretching of starch carbamate and C=O stretching vibrations, respectively. However, the presence of either NH or C=O bands were not observed in dual-modified starch. Furthermore, the combination of electron beam with the hydrolysis of chemical treatment, in dual modification, produced strong degradation in starch structure.
The viscosity of the native starch adhesives showed a direct relationship with the starch concentration. However, the opposite behaviour was observed in both modified and dual-modified starch adhesives. This particularity was attributed to the inability to form polymer networks between starch and water, due to excess degradation in the structure of the polysaccharide. Finally, the improvement in shear resistance was related to the presence C=O group of the starch carbamate. Nevertheless, similar results were observed with starches without this group in their structure.
Future prospective would be focused on testing the effect of microstructure of other lignocellulosic substrates (oak, guayacan, ebony, eucalyptus, teak, pine, etc.) on adhesion mechanism. Further characterization in terms of storage stability, and biodegradability will also be necessary.