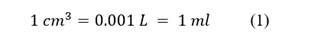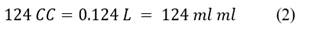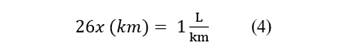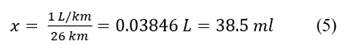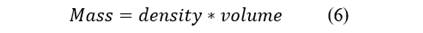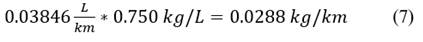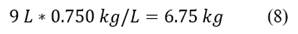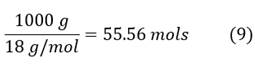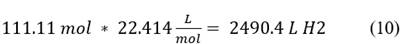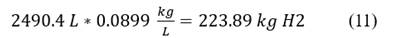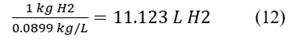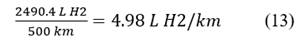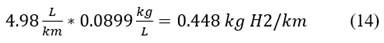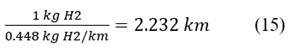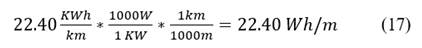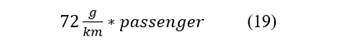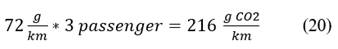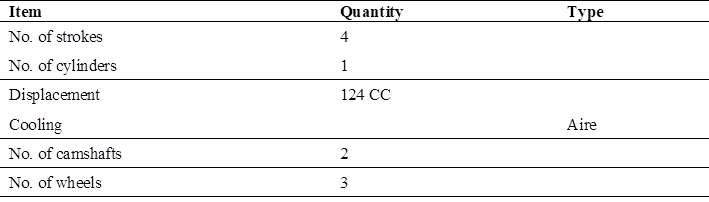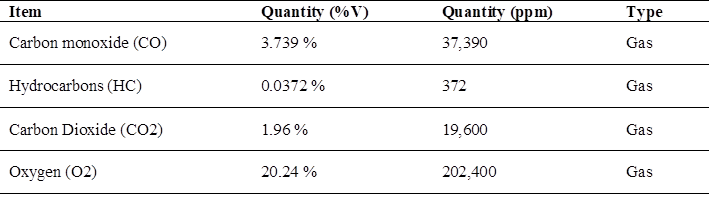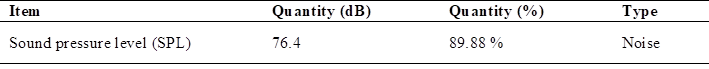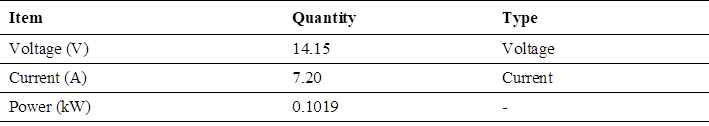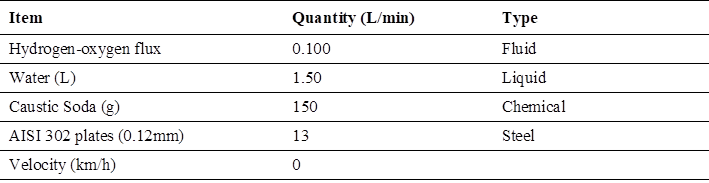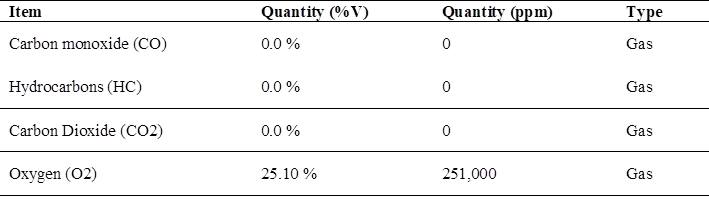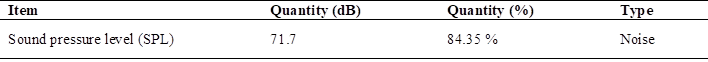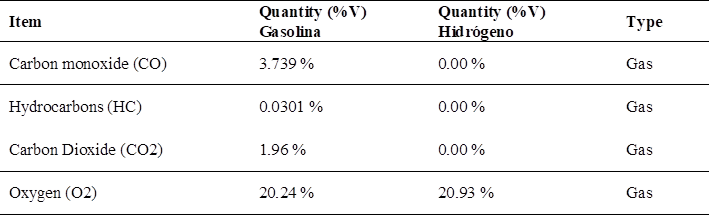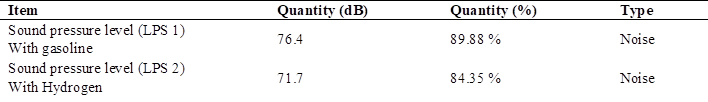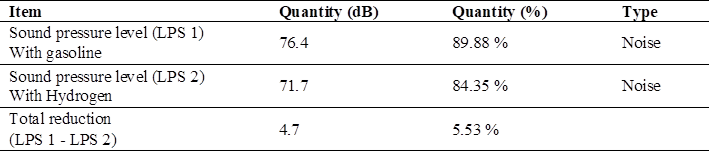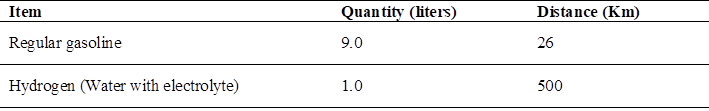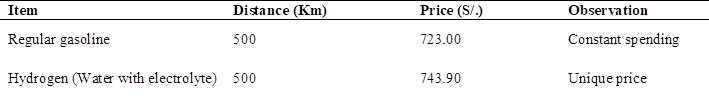I. INTRODUCTION
I n the past, one of the greatest challenges facing humanity has been the reduction of environmental pollution generated by transportation (14).
As an argument for the problem, the use of gasoline in internal combustion engines had several environmental and efficiency problems (21). First, gasoline was a fossil fuel extracted from petroleum, which meant that its production and consumption contributed significantly to climate change. In addition, the burning of gasoline in internal combustion engines emitted a large amount of greenhouse gases, such as carbon dioxide and nitrogen oxide, which contributed to global warming and air pollution (4).
Air pollution had a negative impact on lung development and had been linked to the development of various respiratory conditions such as emphysema, asthma and chronic obstructive pulmonary disease (24). The presence of particulate matter and nitrogen oxide was associated with the development of chronic bronchitis.
In this context, motorcycle cabs, as a means of transport widely used in some regions of the world, had been identified as a major source of toxic emissions. The combustion of gasoline in the internal combustion engines of these vehicles produced a large amount of carbon dioxide, carbon monoxide and nitrogen oxides, among other gases. This problem not only had negative consequences for the environment, but also represented a risk to public health. Lead particles were detected in the air, especially in vehicles that lacked catalytic converters. These lead particles tended to rise to a maximum height of two meters, which meant that we inhaled them directly.
Therefore, it was important to formulate this problem: What effect would the implementation of an electrolyzer in the combustion system of a motorcycle cab have on reducing environmental pollution?
Meanwhile, the reasons why such a project was carried out have been justified. What concerned society and the world at that time was the fight against vehicular pollution caused by internal combustion vehicles. In recent decades, concern about environmental protection had been increasing due to the negative effects that human activity had on the planet. For this reason, it was considered important to look for alternatives to suppress the emission of pollutants in transportation.
One of these alternatives was the implementation of hydrogen as a fuel. The usefulness of this technology would not only contribute to improving air quality, but also to reducing engine noise, which would also help combat climate change and preserve a healthier planet, thus increasing knowledge about how the electrolyzer works.
The impact on engine noise when using hydrogen in a motorcycle taxi can be significant. Hydrogen, when burned in the engine, produces a smoother and quieter combustion compared to traditional fossil fuels. This can result in a reduction in engine noise, contributing to a quieter and more comfortable driving experience for both the driver and passengers. Additionally, noise abatement may have additional benefits for quality of life in urban areas by reducing noise pollution and its potential negative effects on hearing health and general community well-being.
The noise reduction in this research is not only a relevant finding, but also constitutes a significant benefit with important implications. In addition to improving quality of life in urban environments by reducing noise pollution, this noise reduction can contribute to public health by mitigating health problems associated with prolonged exposure to noise, such as stress, fatigue, and sleep disorders. dream. Additionally, it can improve the productivity and general well-being of people living in urban areas by reducing noise interference in daily activities. This noise reduction can also have economic benefits by decreasing costs associated with healthcare and increasing property values in quieter urban areas. In summary, noise reduction not only improves the sound environment, but also has positive impacts on health, well-being and the economy, supporting the importance of this finding in the research project.
The useful life of the engine when using hydrogen in a motorcycle taxi is based on several factors. Hydrogen fuel produces cleaner, more efficient combustion compared to fossil fuels, which can reduce engine wear and debris buildup. This could result in less degradation of engine components, thus extending their life. Additionally, by preventing the combustion of hydrocarbons, corrosive effects and associated wear are reduced, which could further contribute to increased engine durability. However, factors such as engine design, proper maintenance and operating conditions can also influence the life extension when using hydrogen as fuel in a motorcycle taxi.
In addition, this research would benefit workers and users of public and private transportation by reducing transportation costs and improving the quality of the air they breathe. The benefits of this research were significant, as it would considerably reduce environmental pollution and extend the useful life of the engines, increasing the conservation of the exhaust pipe and its component parts.
This research was vital for the automotive and environmental sector, as it would contribute to reducing the amount of pollution produced daily on the planet. Emissions of polluting gases were the main reasons for the decomposition of the protective ozone layer and the increase in greenhouse gases, which affected both human health and the environment in general. Reducing pollution was therefore essential to preserve the planet.
The overall objective was to implement an electrolyzer in the combustion system of a motorcycle cab to reduce environmental pollution. This technology was considered to contribute significantly to the country’s development, improve health and the environment, and benefit all those who use minor vehicles as a means of transportation, whether public and/or private. The specific objectives included the identification of the pollution factors associated with the exhaust gases of a motorcycle cab using 90 octane gasoline, the selection of the electrolyzer as a green hydrogen generator for a 124 CC motorcycle cab, the determination of the exhaust gases generated by the electrolyzer associated with a motorcycle cab, and finally, the comparison of the pollution factors using gasoline and hydrogen in a 124 CC motorcycle cab.
Favorable results were expected that would be of great help to society, the users of minor vehicles as a means of transportation, the vehicle fleet, the environmental sector and the World Health Organization (WHO). The hypothesis put forward in this topic stated that the implementation of an electrolyzer in the combustion system of a motorcycle cab would drastically reduce the environmental pollution generated by carbon monoxide (CO) and carbon dioxide (CO2) (6).
Therefore, it was considered important to promote research and development of this technology for large-scale implementation.
II. MATERIAL AND METHODS
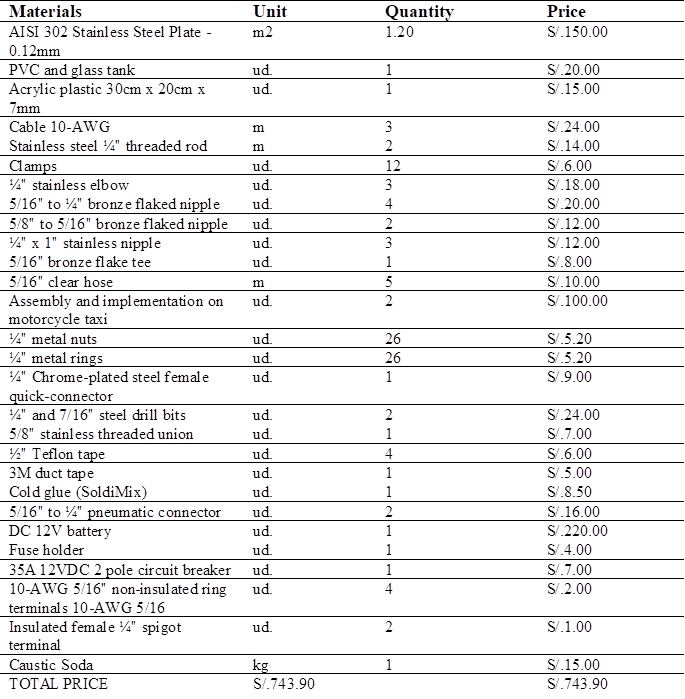
Within the framework of this study, various materials and resources were used in order to facilitate data collection and carry out a comprehensive analysis. The elements that will be used in the development of the research are detailed below. The total cost of implementation, materials and supplies was S/.743.90 Table 1
The nature of the research was applied, since the project focused on the technological field and sought to acquire in-depth knowledge about the operation of the electrolyzer for its implementation in smaller vehicles. Regarding the suppression of polluting agents, it is merely informative, since this approach allowed the development of practical and concrete solutions that addressed the challenge of reducing noise and combating environmental pollution generated by internal combustion vehicles, which at that time at the time it was considered the main source of polluting gases that affected our health and the planet.
The proposed research approach was field experimental, which involved conducting a real-world study to analyze, determine and collect detailed information on the results obtained from real data. This approach allowed a deeper understanding of the phenomena involved in obtaining hydrogen and provided the opportunity to evaluate its feasibility and efficiency under real conditions.
The technique used was both observation and measurement to collect relevant data. Observation involved systematically visualizing and recording the different aspects related to the implementation of the electrolyzer in the motorcycle cab’s combustion system. On the other hand, measurement involved quantifying specific aspects, such as the percentage of exhaust gases allowed in the technical reviews established by the Ministry of Transportation and Telecommunications. The data required for the study included information on the type of fuel used, as this could affect the emissions and the results obtained with the implementation of the electrolyzer. In addition, the performance of the combustion system was analyzed both with and without the electrolyzer installed to evaluate the effects of its implementation on the reduction of environmental pollution.
As a data collection instrument, a guide or data sheet was used, which served as a structured tool to collect relevant information. This guide contained fields for recording data such as the name of the observer, the specific engine being observed, the exhaust gases that polluted the environment, and other specific characteristics.
As procedures, the operation of the motorcycle cab with conventional fuel (90 octane gasoline) was analyzed, and the registration and measurement card were used to collect information on engine performance, including fuel efficiency, exhaust gases generated, engine vibration force and noise impact. It was observed that one liter of gasoline yielded 26 kilometers and with a 9-liter fuel capacity tank, a total distance of 234 km was covered. Therefore, the electrolyzer was implemented to generate hydrogen as fuel to be injected into the motorcycle cab. The electrolyzer worked with a plastic tank that stored 1 liter of water, which was supplied to the electrolyzer dry. Corresponding notes were taken to improve the efficiency and prolong the useful life of the motor, with the objective of avoiding pollution from this minor vehicle, producing water vapor instead of polluting gases. Finally, the results obtained were evaluated, comparing the feasibility, performance, efficiency and gases generated before and after implementing the electrolyzer.
A. Calculation of gasoline fuel per kilometer of driving distance
Cubic centimeters or CC indicate the power of the motorcycle engine. It is the capacity to produce energy and speed (125 CC, 250 CC, 600 CC, etc.) define what is known as engine displacement. Eq. 1
As data we have the calorific value of gasoline being 47.7 MJ/kg, displacement of the motorcycle cab of 124 CC, fuel capacity of 9 liters and distance per liter of gasoline of 26 km. We convert 124 CC to liters and milliliters: Eq. 2)
We use the simple rwule of 3 to decompose: Eq. 3, Eq. 4, Eq. 5
The motorcycle cab consumes 0.03846 L=38.50 ml per kilometer of travel. Eq. 6
We convert the values to kilograms of gasoline, considering that: Eq. 7
234 km of total travel distance is equivalent to: Eq. 8
Note: If the calorific value of gasoline and hydrogen are equal, the same fuel flow will be used. But if the hydrogen is lower, a higher flow rate will be used to move the motorcycle cab.
B. Calculation of hydrogen flow rate in one liter of water
To calculate the hydrogen flow rate, consider the following data of water: Molar mass 18 g/mol, Density 997 kg/m3 equivalent to 1000 kg/m3, boiling point 100 °C and 1 liter of water is equal to 1000 grams. Moles of water in Eq. 9:
As a note: “55.55 moles of oxygen atoms, plus 111.11 moles of hydrogen atoms” (3).
Hydrogen data: Molar volume of some common gases at standard temperature and pressure (0°C and 1 atm pressure).
Eq. 10is the flow rate of hydrogen in 1 liter of water.
We convert to kilograms of hydrogen. How much hydrogen is needed to move the 124 CC, 10 HP, 7000 revolutions per minute (RPM) motorcycle cab?
Some data on hydrogen: Density 89.9 kg/m3, 1 liter of H2 equals to 0.0899 𝑘𝑔 = 0.0899 𝑘𝑔/𝐿 Eq. 11
Eq. 12 is to obtain 1 kg of H2, in liters H2.
C. Hydrogen flow rate and flow per kilometer of run
The electrical conversion efficiency of electrolysis, measured in terms of kilowatt-hours per kilogram of hydrogen (KWh/kg H2), ranges from 60 % to 70 %. This translates into an approximate consumption of 50 to 60 KWh of electrical energy to generate 1 kg of hydrogen. As an additional result, 8 to 10 kg of oxygen is generated for every 1 kg of hydrogen produced (10). “1 liter of water is equivalent to 500 km of travel” (30).
The calorific value of hydrogen is 120.9 MJ/kg.
The flow rate is calculated per kilometer of travel: Eq. 13
In kg H2 would be: Eq. 14
Current required per kilometer of travel: 50 to 60 KWh is equivalent to 1 kg of H2. Eq. 15
To consume 1 kg of H2, it is necessary to travel 2.232 km. Eq. 16
We convert to watts and meters: Eq. 17
We convert to current per meter: Eq. 18
D. Carbon dioxide pollution per passenger and kilometer of traveled route
Calculations are detailed, a motorcycle cab carries 3 passengers besides the driver. Eq. 19, Eq. 20
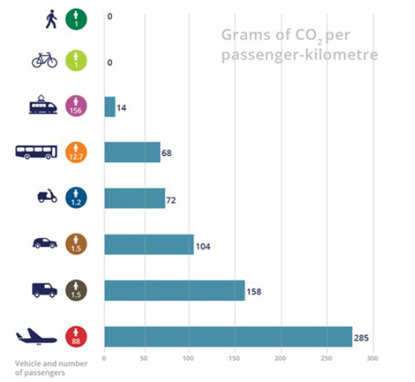
In this calculation, a value of 216 g CO2 per km of travel is given. These values were taken as a reference and can be found in the Fig. 1.
III. RESULTS AND DISCUSSION
For the results obtained, a single-cylinder motorcycle cab of the Itálika brand was used as the object of study based on statistical reasons and representativeness; the data was obtained by observing the motorcycle cab in operation and recorded on an observation sheet.
A. Identification of pollution factors associated with the exhaust gases of a motorcycle cab using 90 octane gasoline
The (Fig. 2), shows the characteristics of the motorcycle cab in an observation sheet, containing parameters such as the title of the project, N° of the sheet, name of the observer, the engine observed which includes the Itálika brand, the FT125 model, the serial N° LLCLPP206BE105056, the industry of manufacture Mexico, N° of plate C2-9318, the date of observation on August 15, 2023, place of observation carried out in the Asentamiento Humano Vista Alegre mz: L. lt: 5 - Nuevo Chimbote and a duration of 40 minutes of observation of the mototaxi. Table 2
In relation to the emissions of polluting gases, the test with the maximum value of pollution with the variation of gases when using gasoline in the motorcycle cab, the data are presented in (Table 3), where it turns out that the data of CO and CO2 are the ones that harm public health.
Note: (Fig. 3) shows the maximum pollutant emission when using conventional gasoline, being the carbon monoxide (CO) value of 3.739 %.
In relation to the sound emissions using 90 octane gasoline, the test with the maximum variation of the sound pressure level (SPL), as data; the noise level allowed in the work in Peru is 85 dB as maximum (15), being 100 % (Table 4).
When analyzing the permitted noise emissions in Peru, the maximum noise pollution value is 76.4 dB, which is equivalent to 89.88 of the permitted level in Peru.
As an interpretation of this result, it is due to the fact that conventional fuel is used, which causes noise pollution and generates health problems such as nervousness, ringing in the ears, among other health problems.
B. Selection of an electrolyzer as green hydrogen generator for a 124 DC motorcycle cab
The design of the electrolyzer is a dry cell, it has 13 AISI 302 stainless steel plates of 0. 12 mm thick, measuring 10 cm long by 15 cm high, the electrolyzer has 9 neutral plates, 2 positive plates and 2 negative plates, the cells work stacked together, with an insulator between plates of rubber material so that they do not make contact and do not generate short circuit; the stacked configuration is 1 negative plate plus 3 neutral plates, successively 1 positive plate plus 3 neutral plates, then 1 positive plate plus 3 neutral plates and finally ends with 1 negative plate; so that the water is a good conductor of electricity and breaks the molecules that make up the water, it is necessary to add caustic soda, in 1. 5 liters of water 150g of caustic soda is added. The electrolyzer has a water capacity of ½ liter, and in the tank or reserve tank with a capacity of 1 liter, uses a working voltage of 12 volts which is supplied by the battery of the motorcycle cab, also uses a 30-amp fuse, 1 two-pole switch to allow the operation of the system, and 1 flame arrester filter safety to prevent accidents from the return of the flame produced by combustion. Hydrogen gas enters through the engine air filter to explode in the combustion chamber of the motorcycle cab.
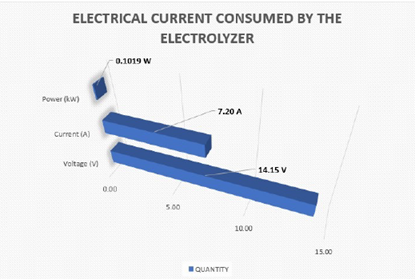
In relation to the electrical energy consumed by the electrolyzer, the test with maximum voltage variation (Table 5).
In this analysis as a characteristic of the electrolyzer, the maximum value of consumption of higher electric current; the data of the electrolyzer are in order of highest consumption: The voltage with a maximum value of 14.15 volts, the current with a maximum value of 7.20 amperes and the working power with a maximum value of 101.9 Watts Fig. 4.
(Fig. 4) shows the current with the highest consumption in the electrolyzer, with a maximum value of 7.20 amperes.
In relation to hydrogen generation, the test with maximum hydrogen flow generated is shown (Table 6).
The analysis of some characteristics and the generation of hydrogen, in this test has in order of higher hydrogen generation with a maximum value of 0.100 liters per minute being 100 ml/min, with a maximum value of caustic soda addition of 150 grams per 1.50 liters of water as maximum value of the electrolyzer, using a speed of 0 km / h (idle).
C. Determination of the exhaust gases generated by the electrolyzer associated with a motorcycle cab
Regarding the emissions of pollutant gases using hydrogen, we have the test with the total reduction of pollutant gases when using hydrogen in the motorcycle cab Table 7, where the CO and CO2 gases tend to 0 % pollution values, being these harmful to health.
In an analysis of the contamination factors using hydrogen, the maximum values of CO and CO2 are equal to 0 %; there is only a maximum value of O2 of 25.10 %.
In relation to sound emissions using hydrogen, the maximum value of the sound pressure level test (SPL), as data; the level allowed in the work in Peru is 85 dB being 100 % as maximum Table 8.
When analyzing the permitted noise emissions in Peru, the tests showed a maximum value of 71.7 dB, equivalent to 84.35 % of the permitted level.
As an interpretation of this result, the sound pressure level is within the permitted range, this is due to the fact that hydrogen is used as fuel, this causes a great noise reduction, generates less noise and avoids alterations such as noise-related health diseases.
D. Compare pollution factors using gasoline and green hydrogen in a 124 CC motorcycle cab
Table 9shows the pollution data to be compared, the amounts in percentage values using gasoline and hydrogen.
In this analysis of the comparison of pollutant gas emissions generated by gasoline and hydrogen, it is found that gasoline has a maximum CO value of 3.739 %, CO2 of 1.96 %; on the other hand, when using hydrogen, the maximum values of CO and CO2 become null or 0.00 %, since there is no pollution at all, and the total pollution of the motorcycle cab is reduced by 100 %.
In the analysis of the comparison of noise emissions using gasoline and hydrogen Table 10, there is a maximum limit of 85 dB allowed in the work in Peru, which is equivalent to 100 %, with gasoline having a maximum value of 76.4 dB equivalent to 89.88% and hydrogen with a lower value than gasoline of 71.7 dB equivalent to 84.35 % of the total allowed.
In this analysis of the reduction of noise emissions using gasoline and hydrogen Table 11, we have the maximum reduced value of 4.7 dB which is equivalent to 5.53 %, thus making less noise than when using conventional fuel by default.
According to the autonomy of the mototaxi Table 12 when using gasoline with a full tank of fuel, its travel range is 26 kilometers in total until the fuel is completely exhausted, since the storage tank is 9 liters.
When using hydrogen, it changes since by using 1 liter of soluble water with electrolyte (caustic soda), the motorcycle taxi is capable of traveling 500 kilometers until all the hydrogen contained in the liter of water is exhausted (30).
As a comparison, the distance that a motorcycle taxi can travel with gasoline is less than what it can travel if it only uses 1 liter of water with electrolyte. Allowing autonomy and being more feasible to refuel with hydrogen than with gasoline Table 13, since not everywhere is a fuel sales service tap. Saving time and money when it comes to moving from one place to another. The only drawback would be to constantly maintain the battery that supplies the electrolyzer.
To travel 26 kilometers, which is 9 liters of regular gasoline fuel, costs 37.60 soles, since gasoline costs 15.81 per gallon. To travel 500 kilometers with gasoline the cost would be 723 soles, if only conventional fuel were used.
Which would be almost entirely the cost of implementing and assembling the electrolyzer capable of generating hydrogen to be used as fuel in the motorcycle taxi.
The cost of assembly and implementation of the electrolyzer has a value of 743.90 soles. The difference being that conventional fuel has to be purchased at storage taps, on the other hand, hydrogen is generated through the electrolyzer, being the only expense after implementation, of the electrolyte, costing an average of 15 soles per kilogram.
The discussion of the results obtained in this research is fundamental to understand their significance and relevance. In this project, an electrolyzer device was designed that has 13 AISI 302 stainless steel plates of 0.12 mm thick, this prototype covers a size of 10 cm long by 15 cm high; the plates were distributed as follows, 2 negative plates, 2 positive plates and 9 neutral plates stacked together, as plate separator was used rubber sheets of 3 mm thick to avoid a short circuit, the device was rectangular in shape and used a battery of 12 volts and 7. 20 amperes, all the plates were fixed on two 6 mm thick blue acrylic bases, measuring 14.5 cm long by 20 cm high. This system takes advantage of the energy stored in the battery, maintaining an average consumption of 7.20 amps. This operation is possible as long as the motor remains running; otherwise, the device stops due to high energy consumption and runs the risk of completely discharging the battery. set of 12 cells, each with dimensions of 8 cm by 12 cm and a thickness of 1 mm, all made from stainless steel. These cells were solidly fixed to a bakelite base with a thickness of 10 mm. The layout included 9 neutral, 1 positive and 2 negative plates, all meticulously insulated with rubber sheets. It should be noted that most of the materials used in the construction of the electrolytic system were obtained through recycling. In addition, they used a 12V - 6A battery. The system takes advantage of the residual or reserve energy stored in the battery, maintaining a constant average current of 2 amps whenever the engine is running. In the absence of this condition, the device stops due to its high energy consumption and the possibility of completely draining the battery charge. All electrolytic devices have been conceived and constructed following the same fundamental principle. Therefore, the inherent characteristics of electrolysis devices will always share a common factor, which is the use of specific materials and a certain form of assembly. However, the variability lies in the number of plates, which is directly related to the size and hydrogen production capacity of each device. Both devices share similarities in terms of using stainless steel plates and 12V batteries. However, they differ in the number of cells, dimensions, plate thickness, type of separators, and in the consideration of the use of recycled materials. In addition, the latter project highlights energy consumption and the need to keep the motor running for constant operation, while the former project does not specifically address this consideration.
In comparison, Diaz et al. (9), designed an electrolyzer with a cell made up of 7 stainless steel plates, each plate has a length of 4.14 cm and a thickness of 1 mm. In this arrangement, 5 plates acted as neutral, one as positive and one as negative, all of them isolated by means of neoprene spacers with a thickness of 3 mm, with the purpose of avoiding short circuits. These plates had a diameter of 9 cm, since the device adopted a circular configuration. In addition, the system was powered by a 12 V, 3 A battery. Overall, both projects share similarities in terms of the energy source and the general objective of generating hydrogen by electrolysis, but differ in several key aspects, such as the number and type of plates, the thickness of the plates, the materials of the separators and the shape of the device. The choice between the two designs will depend on the specific project objectives and desired performance requirements.
As part of this research project, the device incorporated a capacity of 500 ml of electrolyte water in the main source of the system, while 1000 ml or 1 liter of electrolyte water was stored in the reserve tank. In addition, the system was equipped with a 30-amp fuse and a 35-amp circuit breaker, both 12-volt DC and 2-pole. Similarly, the electrolyzer device used a rectangular configuration with 13 AISI 302 stainless steel plates and a 12V battery. These findings are linked to the design proposed by Baltazar (25), which incorporated stainless steel plates with a thickness of 0.15 mm and a capacity of 140 ml in the device chamber. Also, rubber gaskets with a triangular configuration, a contact plate, a fuse, a 12 V relay and a 12 V battery were used. In addition, he suggested three alternative configurations for the device: hexagonal, square or circular. The decision on the shape to be adopted was made considering an analysis of efficiency and economic feasibility, finally deciding on the square shape. Unlike the project established in this research, this alternative device incorporates graphite electrodes, a glass tank and three conduits for the inlet and outlet of both water and hydrogen. In addition, it has a hydrogen tank made of glass, protected by an electromagnetic relay that is connected to the contact plate. The separation between plates is made by aluminum gaskets, and the power supply is carried out by solid wires. In addition, various shapes and materials were considered, highlighting the use of graphite, glass and a protection system with electromagnetic relay.
The results of the project established in this research, using gasoline registered values of 372 ppm of HC, 3.739 % of CO and 1.96 % of CO2; and when using the hydrogen generator electrolyzer, they tend to a total reduction of polluting gas emissions, since using only hydrogen as fuel proves that gases such as carbon monoxide (CO), carbon dioxide (CO2) and Hydrocarbons (HC) tend to values of 0. 0 %, this represents a total decrease of 100 %, being the pollution values null, only giving as a by-product the oxygen coming from the combustion. In comparison, Flórez et al. (29), who used pure gasoline as a fuel source and also combined with hydrogen as an energy source. The researchers recorded concentrations of 344 ppm hydrocarbons (HC), 4.59 % carbon monoxide (CO) and 2.82 % carbon dioxide (CO2) without the application of the electrolyzer system. In contrast, with the introduction of hydrogen as a power source, values of 40 ppm HC, 0.00 % CO and 0.21 % CO2 were obtained. These results represent an 88.37 % reduction in HC, a total elimination of CO and a 92.55 % decrease in CO2, which is highly beneficial for the environment.
As results of the main research, the pollutant gas reduction values are 100 % for the motorcycle cab, while the results obtained by Diaz et al. (9) show that the use of conventional fuel combined with hydrogen led to a reduction in emissions of CO in the range of 13 % to 18 %. In terms of CO2, a reduction ranging from 5 % to 9 % was observed thanks to the electrolyzer device, which is attributed to more efficient combustion. A significant 20 % reduction in O2 emissions was also recorded, as a result of optimized combustion that allowed for better oxygen utilization. Finally, a notable reduction in unburned hydrocarbons was documented, reaching a range of 30 % to 50 %, as a consequence of an improvement and enrichment of the mixture, which contributed to a more effective burning of hydrocarbons through the use of the electrolyzer device.
These findings underscore the diversity of approaches available for hydrogen production, reinforcing the idea that this resource can be obtained in diverse and effective ways.
The conduct of this applied and experimental research study, with the objective of addressing environmental pollution by incorporating an electrolyzer in a single-cylinder motorcycle cab, poses a number of strengths and weaknesses that are essential to understand and evaluate the validity and implications of the results obtained. Among the strengths of this research approach, the high controllability over the study variables stood out. In addition, the reproducibility of the experiments was a significant advantage, demonstrating that the observed effects are consistent and not random. The methodology that was used lends itself particularly well to establishing cause and effect relationships, which facilitated the identification of whether the introduction of the electrolyzer had a direct impact on the reduction of pollutant emissions. In addition, the experiment allowed for accurate and quantifiable data measurements, which were essential when evaluating emissions and other critical parameters. However, it was recognized that this experimental methodology also presented weaknesses that must be considered when interpreting the results. One of the weaknesses was that, despite providing a high degree of control, experiments can sometimes oversimplify reality and not fully reflect real-world conditions. This lack of correspondence with real situations may limit the practical applicability of the results. In addition, the experiment can be costly and consume significant resources, including time and specialized equipment, which may restrict its scope and replicability on a large scale.
This research makes significant contributions compared to other studies of hydrogen implementation in motorcycle cabs, as it addresses multiple key issues related to the feasibility and benefits of using hydrogen as a fuel in this specific context. One of the main contributions of this study is the evidence of a significant elimination of polluting gas emissions by using hydrogen instead of gasoline.
Research has an outstanding relevance both in the scientific field and in the social context in which it is developed. It addresses a current and pressing problem, contributes to sustainability, can influence public policy, and can promote awareness of the importance of reducing environmental pollution. The application of clean technologies, such as the use of hydrogen as a fuel in motorcycle cabs, is essential to address environmental challenges and improve the quality of life in urban environments and beyond.
IV. CONCLUSION
From the results obtained, the following conclusions were reached:
It was possible to implement the electrolyzer in the combustion system of the motorcycle cab, eliminating the environmental pollution produced by the motorcycle cab when using gasoline (conventional fuel), the total removal was 100 % of carbon monoxide and carbon dioxide; and instead of polluting with greenhouse gases, it only produces water vapor through the exhaust pipe, combustion is smoother and quieter; Regarding the useful life of the engine, due to the cleaner and more efficient combustion, the accumulation of waste was reduced, which benefits reducing the wear of internal components and the need for costly maintenance.
Pollution factors associated with exhaust gases were identified using 90 octane gasoline; such as carbon monoxide emissions at a maximum value of 3.739 % and carbon dioxide value of 1.96 %. There were also noise pollution values of 76.4 dB being 89.88 % with respect to 85 dB which are the maximum level allowed at work in Peru
To select the electrolyzer as green hydrogen generator for the 124 DC mototaxi, it was based on the dry cell design, it has 13 plates of which 9 are neutral, 2 positive and 2 negatives; the plates work together with a rubber separator to avoid short circuit in the electrolyzer system, the material of the plates was stainless steel AISI 302 of 0. 12 mm thick, measuring 10 cm long by 15 cm high and using an electrolyte such as caustic soda, which allows better current conductivity in the water.
When implementing the electrolyzer in the motorcycle cab system, it was determined that the polluting gases that cause health problems were suppressed in their totality, since CO and CO2 showed values of 0.0 % contamination and only emitting 25.10 % of oxygen as a maximum value instead of polluting gases.
As a comparison of the contamination factors using gasoline and green hydrogen in a 124 CC motorcycle cab, maximum contamination values with gasoline were 3.739 % of CO, in comparison with hydrogen it was 0.00 % in CO with no contamination at all. Regarding noise emissions, the total reduction using hydrogen was 4.70 dB being 5.53 % less noise than with gasoline.
Unlike previous research that focuses solely on reducing pollutants or improving engine performance independently, this project proposes a comprehensive solution by integrating an electrolyzer into the combustion system. This integration allows multiple problems to be addressed simultaneously, such as reducing polluting emissions and noise, and extending engine life.
This project focuses on motorcycle taxis, although there is research on alternative fuel technologies in larger vehicles, such as cars and buses, this study focuses specifically on motorcycle taxis. This represents a novelty, as this type of vehicle is common in many regions, especially in urban and rural areas of developing countries, where air pollution and noise can be significant, but often overlooked, problems.
It also provides multidimensional benefits; In addition to reducing pollution and noise, the implementation of the electrolyzer aims to extend the useful life of the engine and improve the health of users and local residents. This holistic approach to sustainability and human well-being is innovative and marks a significant difference from previous research that may have focused primarily on one specific aspect, such as emissions reduction.
Finally, in practical application, unlike theoretical or laboratory studies, this project focuses on the practical implementation of the technology in a real operating motorcycle taxi environment. This allows for a more accurate assessment of the feasibility and effectiveness of the proposed solution under real-world conditions of use, representing a significant step forward towards commercial application and widespread adoption of the technology.
1. Manuel Antonio Rodríguez-Perez is an undergraduate student at the Professional School of Mechanical and Electrical Engineering at the César Vallejo University of Trujillo, Peru. Email: roperezma@ucvvirtual.edu.pe, Orcid: https://orcid.org/0009-0003-8786-6009
2. Agustín Pio Estrada-Ramírez has a bachelor’s degree in education from the Professional School of Education of the Catholic University of Trujillo Benedicto XVI, Peru. Email: pioape.5@gmail.com, Orcid: https://orcid.org/0009-0002-6193-9751