Introduction
Multiple sclerosis (MS) is a chronic, demyelinating disease of the central nervous system in which the myelin sheath (insulating covers around the axons) gets damaged in the brain and the spinal cord(1). The underlying cause of the disease is related to genetics and the environmental factors which includes smoking, low levels of vitamin D, obesity and sedentary lifestyle(2). Genetics doesn’t mean that the disease is hereditary transferred; it means that it is caused by some genetic variations in the genes, but some studies have shown a 1-5% familial tendencies for having MS, and the risk even increases up to 25% in monozygotic twins(3).
It is observed that the disease starts appearing in people of 20-40 years of age(4). Caring for patients who are suffering from MS is a real challenge. It is been identified that 2.5 million people are affected worldwide, and amongst them women are affected the most(4). A person with MS lives seven years fewer than someone who doesn’t have the disease(5).
It is in our knowledge that MS doesn’t has a cure, but it is not fatal at the same time. For every person, the care of MS differs. For caring these patients, practical guidelines are developed to select the treatment modality. It is important to note that not every person requires all the care aspects(6).
While we see that the disease is progressive and can lead to disability, there is still not that much literature available to guide the evidence-based practice for all the health care professionals. The absence of enough clinical guidelines leads to great variation in the choice of treatment for caring MS patients. The literature is very limited to few clinical guidelines. Therefore, the purpose of this review was to identify the gaps in the existing guidelines in order to serve as a baseline to propose new guidelines, to identify the available guidelines worldwide, evaluate the quality of the guidelines, and to analyze the levels of recommendations.
Methodology
We performed a narrative review. At first, a literature review was done to identify the clinical guidelines available for MS in all aspects (disease-modifying therapies, diagnostic tests, other treatment options). To find this out, PubMed and Google Scholar were used by looking for keywords “clinical guidelines and multiple sclerosis”, “multiple sclerosis guidelines”, and “practice guidelines and multiple sclerosis”. Then, the clinical guidelines were screened if they were recent (under 5 years). After that, a quality evaluation was done to see if the guideline is worth paying attention to and whose findings are evidence-based. For this, the clinical guidelines were screened if the authors have done or mentioned systematic review, meta-analysis, expert feedback, and levels of recommendations. Those who fulfilled the criteria for a good quality guideline, were then screened to know the questions they answered in their guidelines. For this, summary was developed that includes the guideline questions, and recommendations with their level.
Results
A flow chart with the summary of guidelines found is presented in the figure. During the first step of the review ten clinical guidelines related to MS were found. The names of the clinical guidelines are listed below:
-Practice Guideline: Disease-modifying Therapies for Adults with Multiple Sclerosis (2018)(7).
-ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis (2018)(8).
-MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-establishing disease prognosis and monitoring patients (2015)(9).
-Multiple sclerosis: Management of multiple sclerosis in primary and secondary care (2014)(10).
-Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis(11).
-Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis (2014)(12).
-Guidelines on use of anti‐IFN‐β antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN‐βantibodies in multiple sclerosis (2005)(13).
-EFNS guidelines on the use of neuroimaging in the management of multiple sclerosis (2006)(14).
-EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses (2005)(15).
-Guidelines on the clinical use for the detection of neutralizing antibodies (NAbs) to IFN beta in multiple sclerosis therapy: report from the Italian Multiple Sclerosis Study group (2013)(16).
All these guidelines ranged from the year 2005 to 2018. The second step of the review was to screen the guidelines according to their recency under 5 years. Upon doing this, 6 out of 10 articles were falling under the criteria of 5 years, and correspond to the first 6 first guidelines listed above.
The third step was to evaluate the quality of the guidelines. A good quality guideline refers to the one whose content is evidence-based. The best evidences comes from meta-analysis, systematic reviews, and clinical trial studies (randomized control trials- RCTs)(17). It is possible to give your recommendation a level based on the evidences you get. Therefore, the guidelines were evaluated for the presence of the systematic review, meta-analysis, expert feedback and levels of recommendations. The table shows the evaluation of the clinical guidelines. (Table 1)
Table 1 Summary of the Evaluation of the Clinical Guidelines for Multiple Sclerosis
| Title | Evaluation | |||
|---|---|---|---|---|
| Systematic Review | Meta-Analysis | Expert Feedback | Levels of Recommendations | |
| Practice Guideline: Disease-modifying Therapies for Adults with Multiple Sclerosis (7) | Mentioned about the systematic review | Meta-analysis done for every drug | Expert panel was present to develop the DMT (disease modifying therapy) guidelines | Graded recommendations as level A, B and C |
| ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis (8) | 1. Mentioned that they had done systemic review 2. Mentioned that they used PICO strategy to formulate/ translate the questions | Meta-analysis was done for RCT | Panelist of expert was present for consensus | Graded recommendations as strong, weak and consensus statement |
| MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-establishing disease prognosis and monitoring patients (9) | Experts discussed data from research published in English, and to consider the recommendations contained in previous papers related to the use of MRI in patients with MS. | No meta-analysis mentioned | International panel sat in Spain to discuss the use of MRI in MS patients | No levels of recommendations mentioned |
| Association of British Neurologists: revised (2015) guidelines for prescribing disease-modifying treatments in multiple sclerosis (11) | No systematic review or PICO strategy mentioned | No meta-analysis mentioned | No expert panel mentioned | No levels of recommendations mentioned |
| Multiple sclerosis Management of multiple sclerosis in primary and secondary care (10) | Systematic review was done with PICO strategy | Meta-analysis was done | No expert panel mentioned | No levels of recommendations mentioned |
| Summary of evidence-based guideline: Complementary and alternative medicine in multiple sclerosis(12) | No systematic review or PICO strategy mentioned | No meta-analysis mentioned | Expert panel was selected for the consensus | Levels of recommendations mentioned as level A, B, C and U |
To define the questions of the systematic review, a strategy known as PICO is used.(18). PICO stands for population, intervention, comparison and outcome. As you can see in the table that only two of the clinical guidelines fulfilled the criteria for a good quality guideline. Those two guidelines are:
-Practice Guideline: Disease-modifying Therapies for Adults with Multiple Sclerosis (2018)(7)
-ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis (2018)(8)
It is always considered important for a clinical guideline to be reviewed by a panel of expert before publication for good quality(19). This step also helps the authors to decide the level of recommendation with everyone’s consensus. The use of systematic review has now become a standard in the development of clinical guidelines, still it does not assure us the confidence we have on a particular recommendation on the basis of the evidences, nor does it tell us the applicability. To overcome this issue, a system of GRADE is present(20). GRADE stands for grading of recommendations, assessment, development and evaluation. It is a tool used by the expert panel to decide the grade of recommendation as strong or weak. A strong recommendation is the one with high evidences from the literature and the RCTs, and in which the benefit outweighs the downside. Whereas, if there is balance between the benefit and downside, the recommendation is considered weak(12). According to British Committee for Standards in Hematology (2014):
-Grade A: There is a very low possibility that new research would have an impact on the confidence of the estimated effect.
-Grade B: There is a possibility that new research can change the confidence of the estimated effect.
-Grade C: There is a high possibility that new research would have a significant impact on the confidence of the estimated effect. This recommendation doesn’t hold any evidences from literature, but they come more from the clinical significance.
-Grade U: The recommendation is coming from no evidence.
However, this grading is not universal, and one can define the level of recommendation according to their relevance of the study.
The next step for the review was to develop a summary of these guidelines that includes the questions answered in the guidelines and the recommendations. (Figure 1)
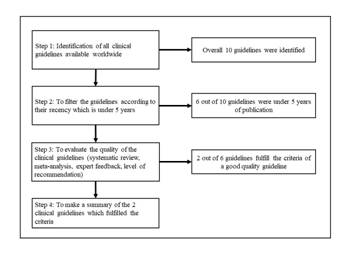
Figure 1 Summary flow chart of the review of Clinical Practice Guidelines related to Multiple Sclerosis.
Practice Guideline: Disease-modifying Therapies for Adults with Multiple Sclerosis (2018)
This guideline basically focuses on the actions of clinicians when dealing with multiple sclerosis patients regarding initiating, switching and stopping the DMTs (disease-modifying therapies). DMTs refers to the medicines that can modify/change the course of the disease(21). There were total 20 recommendations mentioned out of which only 3 of them were Level A. Thirteen of them were Level B and 4 of them were level C. This issue shows us that the confident evidence-based recommendations are very few.
ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis (2018)
This guideline focuses on the overall pharmacological management of the patients with multiple sclerosis that also includes the DMTs. In this guideline, 21 recommendations were mentioned out of which only 3 recommendations were strong. Nine of them were weak and 9 of them were consensus statement. Consensus statement are the ones which are clinically significant based on the expert opinions(22). This guideline also lacks the confident evidence-based recommendations.
Discussion
A good quality guideline does not mean that they follow the same pattern; even the 2 out of 10 clinical guidelines which were of good quality have some variations. Please refer the below mentioned key to refer these two guidelines:
Guideline 1= Practice Guideline: Disease-modifying Therapies for Adults with Multiple Sclerosis (2018) (7)
Guideline 2=ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis (2018) (8)
In one of the guidelines, the strength of the recommendation is graded as level A, B, C and U which is also presented in the result section of the article and it was suggested by the expert panel to grade the recommendations as this. Whereas, in other guideline, the recommendations are graded as strong, weak and consensus statement which was drafted by guideline chairs in a meeting with the methodologists.
It is important to note that only mentioning the fact that you have done systematic review is not enough. In guideline 1, the authors have mentioned that the recommendations are coming from the systematic review of the literature, but they have not provided the readers with a complete set of step by step approach they have taken to suggest the overall recommendations for starting, switching and stopping the DMTs. However, in guideline 2, the authors have mentioned that the recommendations are coming from the systematic review and the randomized control trials, and they have also given the readers with a complete set of information as to what was done on each step.
Furthermore, the guideline 1 narrates that recommendations were agreed upon through a Delphi consensus. Delphi consensus is a method in which multiple rounds of questionnaire is being sent out to the experts, and the answers are then shared amongst the group after each round(23). On the contrary, the guideline 2 narrates that they have used 3 rounds of nominal group technique to reach to the consensus over a recommendation. Nominal group technique is a well-established, structured, multistep group meeting method which is basically use to develop and prioritize the opinions or responses of the questions answered by the experts(24). In medical and healthcare services, three kinds of methods are used to achieve the consensus known as Delphi consensus, nominal group consensus and the consensus development conference(24), and it is up to the team as to what method fulfills their interest best.
The main thing to take into account is the compatibility of the objective with the content of the article. When doing so, it was found out that in guideline 1, the main objective was to formulate recommendations for DMTs in MS patients, yet they have not talked much about the first line therapy which could be used to stop the progression of the disease. The recommendations are more focused on the actions of the clinicians, but not on the drugs particularly. This guideline also highlights the complexity of the decision making when choosing a DMT for a patient suffering from multiple sclerosis. Likewise, in guideline 2, their main objective was to formulate a guideline for the pharmacological treatment of the patients with MS, they have only talked about the DMDs (disease modifying drugs) and not the other categories of the drugs. They could have talked about the symptomatic treatment of the patients with MS because this is also counted as a pharmacological management. Furthermore, in guideline 1, they have mentioned the need to have future researches to improve the decision making for DMTs in MS patients and gave direction in terms of conducting more clinical trials and comparative studies to better the outcomes of the MS patients. On the other hand, guideline 2 does not talk about any of the areas for future researches despite of the fact that only 3 out of 21 recommendations were strong, and thus gives us a clear-cut indication that there is a large room for having more studies to develop the evidence-based recommendations with greater strength.
There is a lot of literature regarding the management of MS, but those are not in cooperated in the form of clinical guidelines. The American Academy of Neurology conducted a systematic review for the literature from 1970-2013 to search for the evidences in rehabilitation management of MS patients. They find out the possible recommendations, but still in need to look upon through future researches(25). The findings from this review could be in cooperated to get a comprehensive view of the MS management.
A systematic review was conducted in Europe published in 2019, to gather the best evidence-based guidelines for the upper limb assessment for people suffering from neurological conditions. They found over 552 records out of which only 34 actually fulfilled the criteria. There criteria for inclusion was also the based on 4 components: systematic review, meta-analysis, expert feedback, and levels of recommendations. This shows that how important these 4 components are for an evidence-based guideline. Out of these 34 guidelines, there were only six guidelines which recommended the specific measures of body structure and function, seven mentioned the global scales without upper limb assessment specifications, and nine guidelines mentioned the importance of global upper limb assessment conducted by trained healthcare professionals. They further mentioned that if the findings of this review would not be given specific attention by the international core set, the data from this review would be wasted.(26)
Another systematic review was conducted in Australia published in 2018, to gather the best evidenced based guidelines for managing patients with stroke and traumatic brain injuries. They had their own inclusion criteria for the studies which included systematic review, meta-analysis and levels of recommendations. They also had a panel of experts who were responsible to choose the recommendations. There were two independent reviewers to select the recommendations. They included 20 clinical guidelines which had approximately 2088 recommendations out of 427 papers available. The important to note here is the fact that they also gave importance to these 4 components. Also, they highlighted the significance of quality guidelines to be included for a better evidence based practice.(27)
It is known that MS leads to decline in the cognitive ability. It is one of the components in the loss of the productive life. There is a need to include the cognitive assessment in the RCTs and clinical, neuroimaging use to learn more about the bases of neural deficits, required to developed interventions coming from a evidence based research(28).
Conclusion
MS is an emerging disease, grabbing everyone’s attention as this disease could be quite disabling if progresses. Surprisingly, there is not enough literature to give us the evidence-based clinical guidelines for the disease. Moreover, the literature which is available, only few of them fulfills the criteria for a good quality clinical guideline. A good clinical guideline is the one which is coming from an evidence-based research, and in which the authors have used systematic reviews, meta-analysis, feedback from the expert panel and allocation of the levels of recommendations. Surprisingly, only 2 out of 10 clinical guidelines falls under a good quality category. This suggests that there are many loop holes in the development of clinical guidelines for caring patients with MS.
Recommendations
We need to work on the development of more practical guidelines to improve the disease outcome, and also to have a uniformity in the treatment modalities. For this, the focus of the research should be on the use of randomized control trials to generate the best evidence-based clinical guidelines for MS patients. Case-control and cohort studies could also be used to get a good quality guideline. These researches could cover the other parts of the management for example, doing assessment, giving medicines, planning rehabilitation measures, and finding more diagnostic measures and treatment modalities for MS patients. If the future researches fulfill the gap which we have in the guidelines, maybe the prognosis of the disease could become better.














